FIGURE 4.
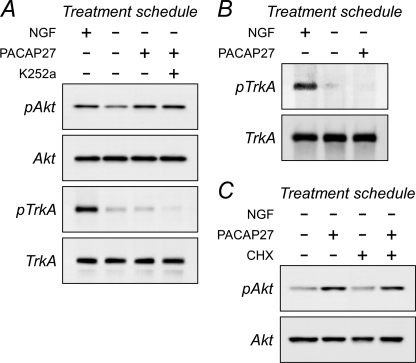
Inhibition of sympathetic receptor tyrosine kinase activity or protein synthesis does not block PACAP-stimulated Akt phosphorylation. A, mature primary sympathetic cultures were acutely withdrawn from NGF and pretreated with 200 nm K252a for 15 min before 100 nm PACAP addition for 3 h. The cultures were harvested in lysis buffer containing phosphatase/protease inhibitors for SDS-PAGE and Western analyses using antibodies for phosphorylated TrkA or Akt. The major form of TrkA in sympathetic neurons is the 140-kDa mature protein. Acute NGF deprivation diminished endogenous TrkA phosphorylation levels. Although the addition of PACAP27 activated Akt, TrkA phosphorylation in the same samples was not enhanced from the low basal levels. Although treatments with the Trk tyrosine kinase inhibitor further diminished culture TrkA phosphorylation, co-treatment with K252a had no effects on PACAP-mediated Akt phosphorylation. B, these results were corroborated in immunoprecipitation experiments. Sympathetic neuronal cultures were acutely withdrawn from NGF to attenuate endogenous TrkA phosphorylation levels (4 h) before PACAP addition for 2 h. The cultures lysed in buffer containing phosphatase/protease inhibitors and the extracts were immunoprecipitated with TrkA antibodies for Western analyses using a phosphotyrosine antibody. PACAP did not stimulate TrkA phosphorylation from the low basal levels. C, similar to experimental designs in panel A, the presence of 5 μg/ml cycloheximide (CHX) for the entire 3-h PACAP treatment period did not abrogate PACAP-stimulated Akt activation. Data are representative of three independent experiments.