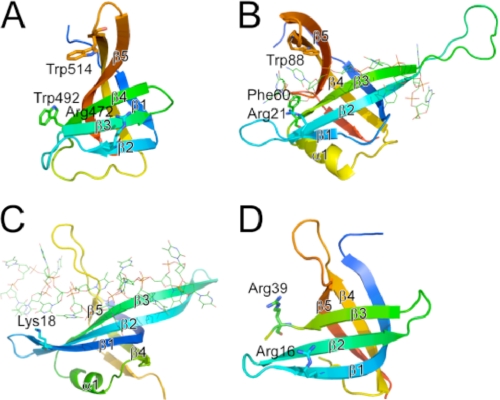
FIGURE 2.
Structural comparison between ttRecJ-OB domain (A) and the OB fold domains of other proteins (B–D). A, region with residues 457–532 of ttRecJ (Protein Data Bank code 2ZXP). B–D, OB fold domains of E. coli SSB (residues 1–112; Protein Data Bank code 1EYG) (B), E. coli PriB (residues 1–102; Protein Data Bank code 2CCZ) (C), and D. radiodurans RecO (residues 2–79; Protein Data Bank code 1W3S) (D). OB folds displayed are colored ranging from blue at the N terminus to red at the C terminus. Displayed residues are assumed to interact with ssDNA. The secondary structure elements that form the OB fold are represented by β1–β5 and α1 according to the literature (22). β1–β5 in (A) and (B) correspond to β15–β19 and β2–β6 in the entire structure of ttRecJ and E. coli SSB, respectively. Residues considered important for binding to ssDNA are shown by sticks. ssDNA is shown by lines.