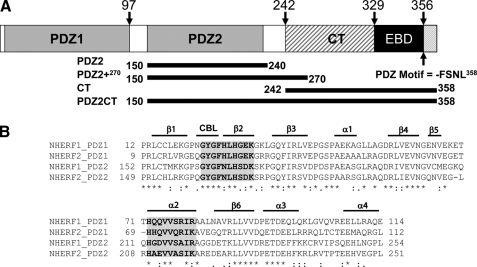
FIGURE 1.
A, schematic representation of the domains of human NHERF1. The C-terminal end of the EB domain contains a canonical PDZ-binding motif. The graph shows the amino acid positions of the differently truncated domains, which include the putative PDZ2240 (residues 150–240), PDZ2+270 (residues 150–270) with the extra C-terminal helical subdomain, and PDZ2CT (residues 150–358). B, sequence alignment of the PDZ domains of human NHERF1 and NHERF2 proteins, annotated with secondary structure elements. CBL, carboxylate binding loop. The residues involved in ligand binding are highlighted in gray and boldface type. The alignment indicates that the sequence in the extra subdomain formed by α3 an α4 is conserved. The alignment was produced in the ClustalX program (66).