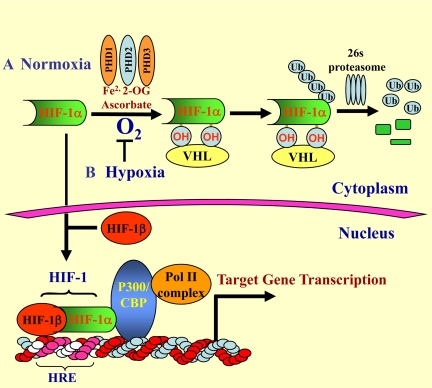
Figure 2.
O2-dependent metabolism of HIF-1α. A: Normoxic regulation of HIF-1α. Under normoxic conditions, the α subunit of HIF-1 is hydroxylated at specific proline residues by prolyl hydroxylases (PHD1-3) that require Fe2+, 2-oxoglutarate (2-OG), and ascorbate. Of these, PHD1 plays a major role in HIF-1α regulation. Once hydroxylated, HIF-1α is recognized and bound by von Hippel Lindau, an E3 ubiquitin ligase; it is subsequently ubiquitinated and rapidly degraded through the 26s proteasomal pathway. B: Hypoxic regulation of HIF-1α. Under hypoxic conditions, HIF-1α is stabilized, translocates to the nucleus, and dimerizes with a constitutively expressed HIF-1β subunit. The dimer associates with several coactivators, including p300/CBP and the DNA polymerase II (Pol II) complex. This protein complex then binds to an enhancer, the Hypoxia-Response Element (HRE), in HIF-1 target genes to initiate gene transcription. Of note, in nucleus pulposus cells, HIF-1α is stabilized even in normoxia.