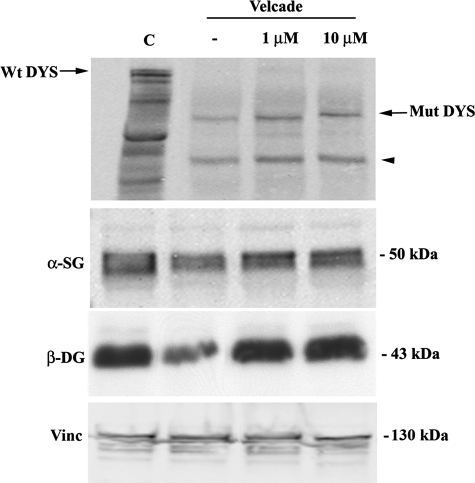
Figure 13.
Inhibition of the proteasome pathway up-regulates protein levels of members of the dystrophin-glycoprotein complex in human muscle explants from BMD patients. Muscle explants were incubated in the absence or presence of Velcade at 1 and 10 μmol/L concentrations for 16 hours. Total protein lysates from control, BMD untreated and Velcade-treated muscle explants were separated by SDS-polyacrylamide gel electrophoresis, transferred onto nitrocellulose membrane, and subjected to WB analysis with specific antibodies against dystrophin (DYS), α-sarcoglycan (α-SG), and β-dystroglycan (β-DG). In the Velcade-group DYS protein levels were significantly increased. Notably, DYS migrated at a molecular weight of 357 kDa that is expected according to the patient’s mutation (black arrow). A lower protein band (approximately 200 kDa) was detected (black arrowhead). This signal could relate to the dystrophin isoform Dp260, migrating at a lower molecular size because of the patient’s mutation. α-SG and β-DG were increased by 30% and 60%. Vinculin (Vinc) was used as a loading control.