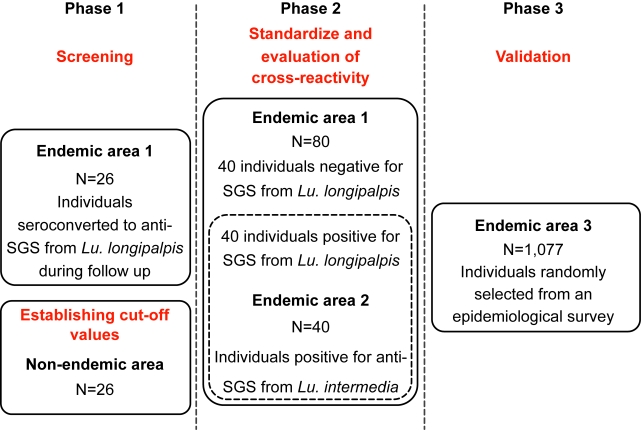
Figure 1. Study flow chart.
The study was divided in three phases, each of them using different sample sets to address the suitability for the use of Lu. longipalpis salivary recombinant proteins as marker of human vector exposure. To establish the cut-off values form the first phase, 26 indiduals from a non-endemic area were tested for SGS and the recombinant proteins (see details in the methods section). In the first and second phases, samples were obtained from São Luis, Maranhão State, in northeastern Brazil, where VL is endemic and Lu. longipalpis is prevalent. To assess cross-reactivity, 40 samples from the VL endemic area who were positive for Lu. longipalpis anti-SGS were tested fom anti-SGS from Lu. intermedia, while other 40 samples from an endemic area of cutaneous leishmaniasis were used (Canoas, a rural village in Bahia, Brazil) were tested for anti-SGS from Lu. longipalpis and the recombinant proteins (dashed line box). The third phase used serum samples obtained from children residing in two other endemic areas for visceral leishmaniasis (Vila Nova and Bom Viver), in Raposa county, Maranhão State, Brazil. The study design details are described in methods. SGS: salivary gland sonitate; VL: visceral leishmaniasis.