Fig. 6.
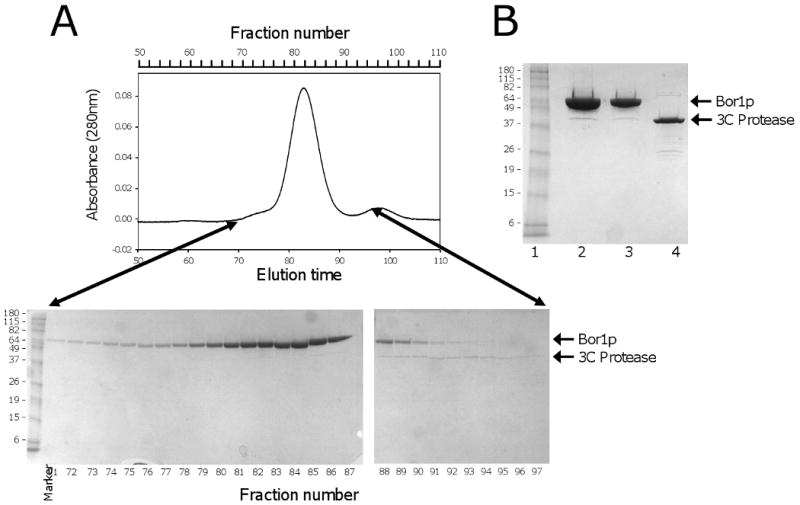
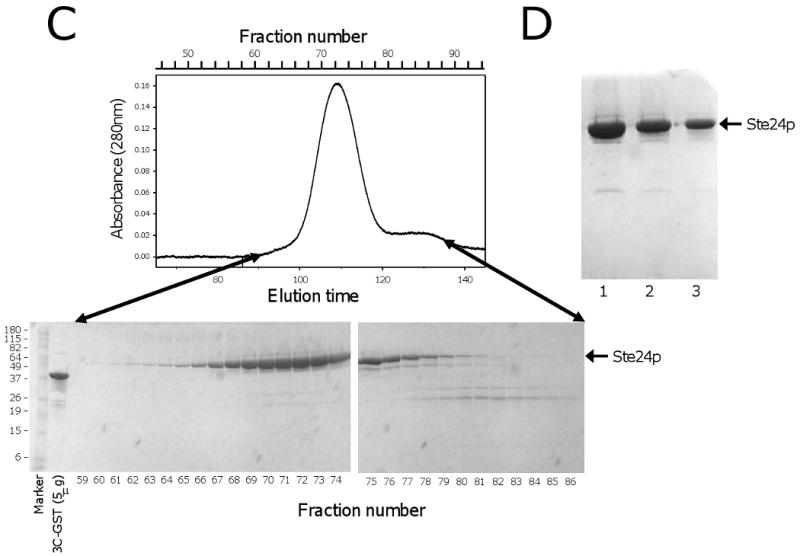
Purification of Bor1p, Ste24p. A)(top) Preparative Superdex 200 gel filtration chromatography during purification of Bor1p expressed from vector pSGP40 and cleaved from IgG Sepharose using GST-tagged 3C protease. The flow rate was 0.8 ml/min. (bottom) SDS polyacrylamide gel electrophoresis of fractions from the gel filtration separation. The molecular masses of marker proteins are indicated in kDa. B) SDS polyacrylamide gel electrophoresis of Bor1p following concentration of the purified protein. Lane 1) Molecular Weight markers; Lane 2) 10 μl purified protein; Lane 3) 5 μl purified protein; Lane 4) 5 μg purified GST-tagged 3C protease. C) Preparative Superdex 200 gel filtration chromatography during purification of Ste24p expressed from the MORF library vector [12] and cleaved from IgG Sepharose using GST-tagged 3C protease. The flow rate was 0.5 ml/min. (bottom) SDS polyacrylamide gel electrophoresis of fractions from the gel filtration separation. D) SDS polyacrylamide gel electrophoresis of Ste24p following concentration of the purified protein. Lane 1) 25 μl purified protein. Lane 2) 12.5 μl purified protein; Lane 3) 6.3 μl purified protein.
