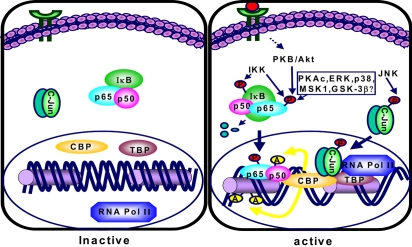
Fig. 3.
Proposed pathways for CBP- and kinase-dependent transcriptional activation of NF-κB and AP-1. A number of extracellular stimuli triggered activation of specific kinases that are involved in transcriptional activation of NF-κB and AP-1. IKK phosphorylates IκB, which results in degradation of IκB by the proteasomes, releasing p65, which then translocates to the nucleus. Some kinases phosphorylate p65, the functionally active NF-κB transactivation subunit. Phosphorylated p65 enhances NF-κB-dependent transcription, probably by influencing the binding affinities of p65 to coactivators such as p300/CBP (CREB-binding protein) or transcriptional initiation complex. CBP is a general coactivator protein that bridges DNA-bound transcription factors to the basal transcription machinery. In addition, CBP, having intrinsic acetyltransferase activity, acetylates p50 (NF-κBκDNA binding subunit). This causes increased affinity of NF-κB to DNA. On the other hand, JNK-induced phosphorylation of c-Jun, a component of the AP-1 complex, facilitates its interaction with p300/CBP. Abbreviations: GSK-3β,..glycogen synthase kinase-3; IκB, Inhibitor of NF-κB; MSK1, mitogen- and stress-activated protein kinase 1; P, phosphate; PKAc, catalytic subunit of protein kinase A; PKB/AKT, protein kinase B; JNK, c-Jun-N-terminal kinase; AP-1, activator protein-1.