Abstract
Sporadic outbreaks of epizootics including SARS coronavirus and H5N1 avian influenza remind us of the potential for communicable diseases to quickly spread into worldwide epidemics. To confront emerging viral threats, nations have implemented strategies to prepare for pandemics and to control virus spread. Despite improved surveillance and quarantine measures, we find ourselves in the midst of a H1N1 influenza pandemic. Effective therapeutics and vaccines are essential to protect against current and future pandemics. The best route to effective therapeutics and vaccines is through a detailed and global view of virus–host interactions that can be achieved using a systems biology approach. Here, we provide our perspective on the role of systems biology in deepening our understanding of virus–host interactions and in improving drug and vaccine development. We offer examples from influenza virus research, as well as from research on other pandemics of our time – HIV/AIDS and HCV – to demonstrate that systems biology offers one possible key to stopping the cycle of viral pandemics.
Keywords: antiviral therapeutics, genomics, metabolomics, next-generation sequencing, proteomics, systems biology, vaccines, viral pandemics
Infectious diseases caused by viral pathogens pose significant threats to global health. Currently, the world is faced with a number of pandemics attributed to influenza virus, HCV and HIV. Influenza virus has caused four major pandemics during the 20th century of which the 1918–1920 pandemic was most severe and estimated to have killed 50–100 million people worldwide [1–3]. Nations have implemented pandemic preparedness strategies to create a global front against emerging infectious diseases. Despite existing preventive measures, the current H1N1 influenza pandemic demonstrates the potential for emerging influenza viral threats to quickly surpass these measures and become a pandemic. To date, the CDC has reported nearly 9000 hospitalized H1N1 cases in 2009 from USA alone and there is growing concern over the rise of a more deadly strain coinciding with the regular flu season. In addition to recent events with the influenza pandemic, it is important to note that nearly 40 million people globally are currently living with HIV, while HCV infects over 170 million people worldwide and approximately 30% of those who are chronically infected develop liver fibrosis and hepatocellular carcinoma. It is necessary to thoroughly understand virus–host interactions if we are to address current and future viral pandemics, and this may best be accomplished by taking a systems-level approach in researching virus infection.
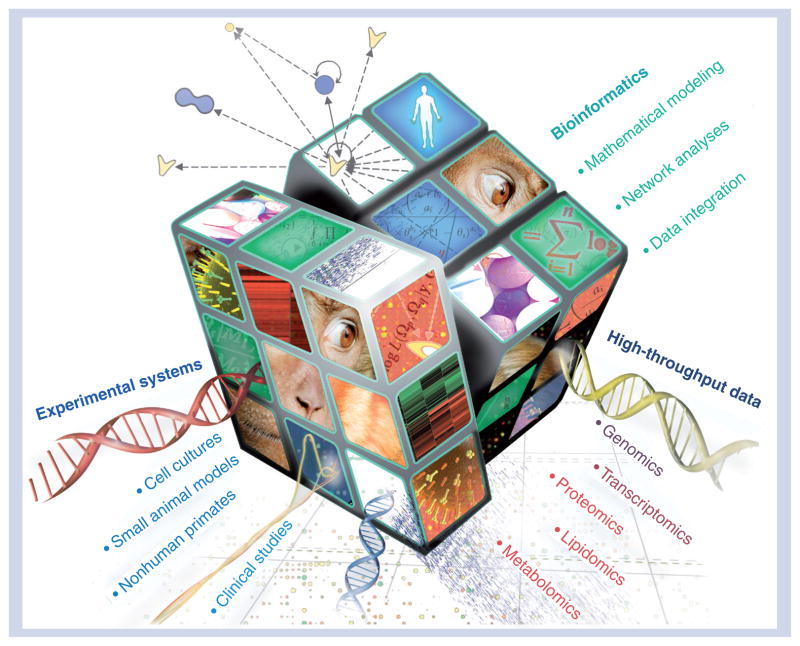
Systems biology is a global network analysis that utilizes sophisticated computational algorithms and data from high-throughput technologies to make predictive models. These models are then assessed by introducing perturbations within a given cellular network and monitoring the responses, thereby relating alteration of specific components to the system as a whole. Biological validation of the predictions using a variety of experimental systems allows current models to be refined and also provides the basis for further predictions and hypothesis generation. The Rubik’s cube illustrated in Figure 1 symbolizes systems biology as a multidimensional matrix integrating computational models, experimental systems and high-throughput data types in a variety of permutations to solve the puzzle of virus–host interactions. Such efforts are critical to researchers’ objectives to identify and prioritize potential host-cell factors for novel antivirals. This is a time of great opportunity for biomedical researchers as better technologies and more integrated approaches will lend new perspective to some of the most trying viral challenges of our time. Systems biology will probably reinforce current pandemic strategies by aiding in the development of effective therapeutics and vaccines. This is especially important to mitigate emerging viral threats and to hopefully help bring us toward a resolution to the H1N1 influenza, HIV/AIDS and HCV pandemics.
Figure 1. Deciphering virus–host interactions using systems biology and implications for therapeutic development.
Representation of systems biology as an interdisciplinary field integrating data from diverse high-throughput measurements, experimental systems and computational models to help solve the puzzle of pandemic viruses and aid in the development of antiviral therapeutics and effective vaccines.
Host biomarkers & genetic determinants of infection
Innate immune host defenses are triggered in response to virus infection. While failure to induce innate immunity is likely to result in increased infection, an overly aggressive response can exacerbate disease severity, as observed with 1918 pandemic influenza virus infection. Our laboratory has used genomics to profile transcriptional changes in 1918-infected respiratory tissues from mouse and nonhuman primate (NHP) animal models. These demonstrate a strong and early activation of innate immune pathways – coupled with enhanced expression of inflammatory and cell death-related genes – and, remarkably, the host response is sustained throughout the course of infection [4,5]. The assessment of host response using functional genomics is a discovery-driven approach that has revealed critical information in the regulation of gene expression pertaining to innate immune responses. By probing deeper into virus–host interactions that occur early after infection, it will probably reveal more information regarding disease progression.
To obtain a more complete picture of virus infection, genomics is being integrated with proteomics, which together offer greater coverage of cellular and molecular events occurring during viral replication. Proteomic analysis provides information on relative changes in protein abundance and details regarding post-translational modifications, such as protein phorphorylation and ubiquitination, which can influence effector function and, possibly, infection. Comparative genomic and proteomic analyses have revealed correlations between gene expression and relative protein abundance in a NHP model of influenza infection, particularly with respect to interferon induction. These analyses implicated ‘molecular signatures’ in the periphery that could signify molecular events of pulmonary influenza infection. Identification of innate immune-related genes coregulated in the lungs and periphery broadly correlated with MX1, IFIT1 and STAT1 immune mediators that were detected in the lungs [6]. Similar studies can serve as a foundation for systems biology approaches to further characterize virus-induced cellular perturbations in the host and, ultimately, identify biomarkers that can be used to detect disease and predict infection outcomes. This information will be particularly useful for measuring exposure if it can be extrapolated to biomarkers in easily accessible fluids, such as blood. For example, immune mediators identified in the blood of vaccinated individuals that can predict vaccine immunogenicity would be informative biomarkers ensuring healthcare workers responding to an outbreak and individuals in and around an epicenter are fully protected. Furthermore, altering standard HIV testing to include biomarkers of early infection may be advantageous – particularly among individuals who develop HIV antibodies more slowly – as earlier diagnosis could translate to earlier initiation of behavioral modifications and antiviral regimens.
Similar virus exposure does not have the same impact on all individuals and this variability is in part due to genetic differences. It is essential to consider host genetics when developing biomarkers. The integration of systems genetics into systems biology will allow us to better assess the impact of genotype, concurrent physiological factors (e.g., comorbid conditions, age and gender) and environmental exposures (e.g., diet) on viral infection both separately and in concert. For example, it has been shown that experimental manipulation of exposure to methyl-donor micronutrients in pregnant dams can alter the development of offspring through the impact of the micronutrients on epigenetic modification of DNA [7]. Given the potential impact of epigenetics on disease, such studies reinforce the importance of studying intrinsic and extrinsic factors concurrently within the framework of a systems biology approach.
Ultimately, integration of systems genetics into systems biology could help in predicting those individuals who are more susceptible to disease, and who respond better to specific therapies. Investigation of host genetics is expanding with better animal models. The Collaborative Cross (CC) project is currently developing a panel of mice with genetic diversity that will more closely mirror diversity in human populations [8,9]. Current studies examining the response to SARS and influenza infection between the parental strains of the CC project indicate a range of phenotypic response to infection between mice with different genetic backgrounds. There is marked distribution in weight loss among mice of different genetic background following infection with these viruses [Baric R, Pers. Comm.]. Closer interrogation of the genetic components dictating these responses will provide important insights into phenotype–genotype relationships in infection.
Improved animal models may also help us in understanding and interpreting genotype associations with HIV. This is important as success in determining the host-genetic components to HIV has been relatively slow using the current techniques in isolation. Targeted investigation of CCL3L and CCR5 genotype and genome-wide association studies have indicated an association between HIV infection and variation at CCL3L/CCR5 genes [10] and HLA alleles [11,12], respectively. Subsequent targeted investigation of these genetic variants report that CCL3–CCR5 genotype impacts immunity during highly active antiretroviral treatment in HIV patients [13], and may be useful in conjunction with standard clinical markers to predict risk of AIDS in HIV patients [13,14]. Studies specifically examining alternate non-HLA genetic variants and HIV have at times indicated that reported associations for these variants may be largely driven by their linkage to HLA [15]. Furthermore, associations between genotype and HIV may depend on confounding factors. This is highlighted by studies that demonstrate the relationship between variants at cytotoxic T-lymphocyte genes and HIV depend on time and the cohort examined [16]. With systems biology approaches, we may be able to uncover the mechanisms underlying these associations.
New horizon for drug development
RNA viruses are difficult targets for drug development because they have relatively small genomes, presenting a limited number of viral drug targets, and their high frequency of mutation often results in drug resistance. The effectiveness of targeted Tamiflu treatment and prophylactic measures in containing emerging influenza viruses is largely overestimated owing to differences in patient response to drug treatment and diminished drug efficacy against escape mutants that arise in response to therapy. For example, single amino acid substitution, H274Y, in the influenza glycoprotein neuraminidase is sufficient to confer Tamiflu resistance in highly pathogenic avian influenza viruses [17]. Recently, this mutation was detected in clinical isolates from H1N1 pandemic virus-infected patients receiving Tamiflu treatment, as well as in an individual who had not undergone drug therapy. The latter occurrence is especially problematic as we are continuing to see an increased prevalence of circulating human influenza variants harboring the H274Y mutation in the absence of drug selection [18].
Similar challenges occur with AIDS therapies, and HCV treatment options have only recently advanced with the development of telaprevir [19,20]; however, current therapeutics merely manage HIV and HCV infection at best. Limitations of using a structure-based drug-design approach merits expansion of antiviral research efforts to include cellular targets that may be exploited for therapeutic intervention. As proof of concept, pharmacological cyclin-dependent kinase inhibitors R-roscovitine [21] and flavopiridol [22] have been shown to effectively block Tat-activated transcription of the HIV-1 long-terminal repeat promoter. Genomic and recent proteomic analyses from our laboratory have demonstrated virus-induced perturbations regulating cell-cycle pathways coincide with optimal viral protein production [23]. This implies HIV- and HCV-mediated effects in cell-cycle dysregulation are advantageous for viral replication, perhaps by prolonging the progression of cell death. Information unveiled from these studies presents further opportunity to investigate novel cell cycle-related targets, as well as interrogation of additional cellular networks including ubiquitination and nuclear transport pathways. Furthermore, Chan and coworkers reported that proteome changes detected in HIV-1-infected primary CD4+ T cells during peak viral replication are largely abolished in the presence of a non-nucleoside reverse-transcriptase inhibitor, efavirenz, demonstrating that evaluation of antiviral effects during infection can be functionally validated with proteomics [24].
Additional options for therapeutic targets from a host perspective include development of Toll-like receptor agonists, such as CpG oligo-deoxynucleotides that can influence Toll-like receptor 9 responsiveness in cells to enhance downstream immune signaling pathways, which has been evaluated in HIV-1-infected individuals [25]. Moreover, a drug class of antagonists that includes antiretroviral maraviroc – a CCR5 antagonist that disrupts HIV-1 entry by blocking the envelope glycoprotein gp120 interaction with the cellular coreceptor – has been shown to be the best treatment option for drug-resistant patients [26]. This highlights the potential to further develop therapeutics that antagonize viral protein function.
Systems biology can overcome obstacles faced with current drug-development methods by helping to realize the potential of a host-oriented drug-discovery paradigm [27], as conceptualized by the Rubik’s cube shown in Figure 1. Only a small fraction of the countless cellular pathways and host factors involved in viral replication have been characterized. This makes it a daunting challenge to identify an effective cellular target(s) that will have the greatest impact on infection treatment. Large-scale screens have uncovered novel host factors that are involved in HIV-1 replication, such as Golgi transport proteins Rab6 and Vps53 and karyopherin transportin 3 necessary for HIV-1 viral entry and nuclear transport, respectively [28]. An RNAi screen in Drosophila yielded a handful of host genes including COX6A1, a cytochrome c oxidase, and NXF1, nuclear RNA export factor 1, important during influenza virus replication [29]. Protein–protein interaction data can provide biochemical validation of virus–host interactions and, together with integrated ‘omic’ data types, will aid in network analyses. Constructed cellular interaction networks can be enriched by screening data sets against databases, for example, the HIV-1 Human Protein Interaction Database established by the National Institute of Allergy and Infectious Diseases (NIAID), can be modeled to reveal suspected components for targeted perturbation analyses.
In comparing networks between sublineages of pandemic influenza viruses or even across viruses to include HIV and HCV, we may be able to perhaps expose the Achilles heel common to multiple viral pathogens. Recently, small molecule therapeutic, FGI-106, revealed inhibitory activity against multiple hemorrhagic fever viruses as well as HIV-1 and HCV, suggesting that the compound probably targets a host factor required for replication by several viral pathogens [30]. This demonstrates the promise of identifying a host factor or pathway common to distinct viruses. Cellular factors involved in vesicle biogenesis may also present an opportunity to develop novel therapeutics exhibiting broad-spectrum antiviral activity. For example, by modulating endosomal sorting machinery, such as Tsg101 or host factors from the endosomal sorting complex required for transport (ESCRT) pathway, the production of multiple viruses may be interrupted. Enveloped viruses including HIV-1 and Ebola hijack these factors to facilitate viral budding, and studies have implicated a role for tumor susceptibility gene 101 in the replication of multiple seasonal influenza viruses (e.g., H1N1, H2N2 and H3N1), as well as highly pathogenic avian influenza H5N1 virus [31]. Thus, the use of systems biology approaches for antiviral research would translate into effective therapeutics, with the potential to provide the greatest preventive measure against impending pandemic threats.
Effective vaccines: a distant hope?
Vaccination is the best strategy for prevention and control of viral pandemics. We lack vaccines for both HIV and HCV and a large shortcoming with the influenza vaccine is its inability to induce broad-spectrum immunity against a wide range of influenza A subtypes, requiring repeated immunizations. The need to develop effective vaccines to protect against newly emerging influenza strains is a global health priority; however, as we cannot effectively predict pandemic influenza strains that will emerge, it is near impossible to adequately prepare an effective vaccine in anticipation of the next influenza pandemic. The time delay from when a pandemic influenza virus first emerges to vaccine distribution is a major barrier to control of virus spread. Researchers have concentrated much effort in generating an experimental swine-origin H1N1 vaccine, and anticipate having a commercial vaccine available in time for the upcoming flu season.
It is unclear as to what causes a vaccine to be successful. All we really have are correlations. To characterize the constituents of a successful immune response, an understanding of the molecular signatures predicting strong and persistent humoral and T-cell responses in the host is imperative. We contend that genomic signatures of the innate immune response cannot only be used as biomarkers, but will also truly reveal mechanisms associated with successful vaccinations. Transcriptional profiling following vaccination of macaques with a live-attenuated influenza A/Texas/36/91 (H1N1) virus containing a truncated NS1 gene coding for the first 126 amino acids (TX91 NS1Δ126) demonstrates strong innate immune responses associated with interferon induction and enhanced gene expression related to T- and B-cell receptor activation and dendritic cell maturation [32]. This study also demonstrates the potential to use functional genomics to assess vaccine prototypes, for instance, a truncated NS1-stimulating antigen, in order to determine vaccine candidates that elicit the greatest innate immune response.
HIV vaccine research has also been stymied owing to the lack of immune correlates that define infection. Despite exhaustive efforts, we still lack a vaccine against HIV, and the failure of the Merck STEP trial is viewed by some as a crushing defeat for HIV vaccine research [33]. A great challenge in HIV research is to understand what differentiates natural host infection from non-natural hosts, as well as immune correlates predictive of vaccine-induced protection. In macaques, SIV infection is pathogenic leading to the progression of AIDS, while African green monkeys (AGMs) pose a restriction to disease exhibiting only mild infection. To understand the molecular differences that underlie the mechanism of host restriction to SIV infection, transcriptional profiles were compared between nonpathogenic (AGMs) and pathogenic (Pigtailed macaques) animals during the acute phase of SIV infection [34]. The innate immune response differentiating these two SIV-infection models could provide useful biomarkers associated with successful vaccination. In a recent companion study, one distinction noted between pathogenic and nonpathogenic SIV infections was an imbalance between T-cell subsets. In macaques, there was a loss of proinflammatory-producing Th17 cells during the course of infection, while AGMs maintained levels of Th17 and Treg populations that were even found to increase during the course of infection [35]. If an AGM-type response can be achieved in vaccinated people, then perhaps this might be indicative of a successful vaccine.
The potential of a systems approach in vaccine evaluation has been suggested by studies investigating the yellow fever virus. A recent yellow fever vaccine study presented by Querec and coworkers related cytokine and genomic data sets from peripheral blood mononuclear cells of vaccinated individuals to the highly immunogenic nature of the vaccine. Several molecular signatures identified from their transcriptomics analysis correlated with CD8+ T-cell responses to the yellow fever vaccine (YF-17D) [36]. Similar associations were observed in a yellow fever vaccine companion study presented by Gaucher and coworkers [37]. However, contrary to the title of the study by Querec et al., a true systems biology approach was not taken into account in evaluating the immunogenicity of YF-17D. If computational analyses modeling T-cell and humoral cellular networks with vaccine-induced host responses had been performed in either of these studies, the authors would then have been closer to a true systems biology approach. In doing so, they would have been able to interrogate the cellular pathways and selected perturbations within these networks to more clearly define immune correlates of vaccine immunogenicity. Gene-expression analysis from YF-17D and TX91 NS1Δ126 vaccine trials implicate early signatures of innate immune activation as having predictive value for subsequent phenotypic outcomes for these two unrelated viruses. Thus, although systems biology has yet to be fully applied to vaccine research, the approach holds great promise in identifying more concrete biomarkers of successful vaccination and, possibly, immune correlates for multiple viral pathogens.
Conclusion & future perspective
The future of systems biology for pandemic research depends on extending it into more complex eukaryotic systems, such as primary cell culture, small rodent and NHP models. Success of systems biology has been achieved almost exclusively in simpler models including Saccharomyces cerevisiae and Caenorhabditis elegans, and the field has remained within the realm of these organisms because more complex eukaryotic systems are less well characterized and exhibit greater variability. Nonetheless, systems biology is evolving and emerging technologies, such as next-generation sequencing (NGS), may encourage adoption of systems biology approaches in more complex models since it has the potential to greatly enrich current data sets by allowing us to study novel splice variants, noncoding RNA and sRNAs. Despite the attention these RNAs have received for their potential role in infection and other diseases (reviewed in [38,39]), novel versions of these RNAs are not typically quantified using traditional microarray techniques. NGS also provides the opportunity for better genomic and transcriptomic characterization of less well-defined genomes of NHPs, and the discovery of novel mutations on a genome-wide scale. However, the use of NGS information has challenges, including technical issues with library creation and computation issues stemming from sequence fidelity, particularly at the end of short reads.
Clearly, the extension of systems biology to more complex models is vital in understanding the true complexity of host–virus interactions, but in order to do so, it will require more sophisticated computational models to deal with the increasing levels of complexity. To improve our understanding of these complex systems, a greater breadth of high-throughput data may be required in order to help characterize these models. Metabolomics, including lipidomics and glycomics, among others, hold promise for assessing the host response to infection. Metabolomic data will give additional information regarding pathway activation and signaling during viral infection. Metabolomics technologies aim to identify and quantify the broad repertoire of small-molecule metabolites (e.g., metabolic intermediates and hormones) that are present in an organism. Since these chemical fingerprints represent the end products of gene expression, they serve as a functional readout of phenotype and provide a more complete picture of the biology of an organism and its response to perturbation. This rapidly emerging field is receiving considerable attention in the virology community. The integration of transcriptomic and metabolomic data has led to the observation that human cytomegalovirus institutes its own specific metabolic program, which occurs, in part, by virus-induced transcriptional changes that modulate the accumulation of select glycolytic, citric acid cycle and pyrimidine nucleotide biosynthetic intermediates [40]. Subsequent global kinetic flux measurements identified a dependence on increased nucleotide and fatty acid biosynthesis and further demonstrated that pharmacologic inhibition of lipogenic activities impaired viral replication [41].
The collective integration of transcriptomic and/or proteomic and metabolite data provides valuable insights into the metabolic interplay occurring during HIV and HCV infection and the potential implications for viral pathogenesis [Diamond DL, Syder AJ, Jacobs JM, Sorensen CM, Walters KA: Temporal proteomic and lipidome profiles reveal hcv-associated reprogramming of hepatocellular metabolism and bioenergetics. PLoS Pathog. (2009), Submitted] [23,24,42–45]. For example, we have identified profound modifications in the transcriptome, proteome and metabolome that are predicted to contribute to HCV-induced liver damage via a series of events involving marked disruptions in cellular metabolic homeostasis, perturbation of the cell cycle in response to increasing cellular stress and impaired host-cell defenses [Diamond DL, Sydner AJ, Jacobs JM, Sorensen CM, Walters KA: Temporal proteomic and lipidome profiles reveal hcv-associated reprogramming of hepatocellular metabolism and bioenergitics. PLoS Pathog. (2009), Submitted] [44]. Similarly, metabolome analyses of cerebrospinal fluid from SIV-infected macaques facilitated the identification of increased lipid species during infection-induced encephalitis and subsequent transcriptomics studies further uncovered a mechanistic link to increased phospholipase activity [45]. Of further interest is a recent study describing plasma metabolite changes that track the innate immune response occurring during murine infection with lymphocytic choriomeningitis virus and provide a potential link between altered metabolites and virus-induced oxidative stress [46]. These studies demonstrate the potential of functional genomics technologies to provide a global view of the host cell response to virus infection, thus identifying candidate regulatory targets for molecular diagnostics and therapeutic intervention. These efforts have led to the identification of previously undescribed protein and lipid bottlenecks that are predicted to play a key role in HCV-associated metabolic reprogramming, and the identification of miRNA–mRNA regulatory modules that may play an important role in post-transcriptional control of apoptotic gene expression during HCV infection [45,47].
In an effort to further integrate our omic approaches, we have begun to augment our functional genomics analyses with computational modeling and informatics activities aimed at constructing a molecular interaction network that describes the relationships between major host-cell response processes over time and relates these response pathways to infection outcome (Figure 1). It remains a significant challenge to integrate the vast amounts of data collected from these complimentary high-throughput functional genomics studies into a single systems-level view that describes the behavior of a system in response to perturbation. One significant challenge to this type of research is the highly computationally intensive nature of the modeling aspects of the approach. To overcome this challenge, greater collaboration across biological and computation disciplines will be required for the most signifi-cant gains in the field to be realized. Gaps in metabolomic and proteomic data annotation, and inconsistent correlation between data types (e.g., genomic and proteomic) complicate data analysis and interpretation and, therefore, limit the use of these technologies. As better methods are developed to identify and characterize data output and link data to function, these issues will become less important.
The use of systems biology in complex models will present a comprehensive picture of virus–host interactions. Information from this approach will effectively culminate in providing the knowledge, drugs and vaccines to prevent the next pandemic or emerging virus threat. As we are being ushered into a new era of personalized medicine wherein all genetic and environmental exposures are considered in the development of individual treatment regimens, we can expect systems biology to enhance patient treatment options by providing more tailored drug regimens based on individual responses to therapies. At the moment, systems biology may seem to be taking the road less traveled. Yet, as Robert Frost aptly stated, that has made all the difference.
Executive summary.
Biomarkers that can serve as molecular indicators of infection
Systems biology is an interdisciplinary field that uses computational modeling to integrate information generated from high-throughput technologies and experimental models, which can provide a deeper understanding of virus–host interactions in complex systems.
Transcriptional profiling demonstrates that innate immunity plays an important role in host defense against virus infection.
Clinical assessment of genetic information will guide the development of better biomarkers of infection, and understanding of individual variability in susceptibility to infection and response to therapies.
Better animal models, such as the collaborative cross mice, are an important resource for studies of host genetics.
Development of antiviral therapeutics requires closer inspection of virus-induced perturbations in the host
A new paradigm in drug development is required for more effective antiviral therapeutics.
Systems biology may reveal novel drug targets by aiding in the identification of host factors and cellular pathways essential for viral replication and that may be common to multiple viruses.
Agonists that enhance innate immune responses and antagonists that competitively inhibit virus–host interactions will serve as attractive candidates for inhibitor design and development.
Correlates of immunity for successful vaccination
HIV and HCV vaccines and more effective influenza vaccines are needed to protect the public from emerging and ongoing viral pandemics.
Gene-expression profiles distinguishing natural hosts from non-natural hosts during SIV infection will likely aid in the design of future HIV vaccine prototypes.
Determination of ‘molecular signatures’ of early host responses, including innate immunity, which are associated with successful vaccination will lead to improved prediction of humoral and adaptive immune responses.
Systems biology is an evolving field
Greater integration of new technologies including metabolomics, lipidomics and glycomics, into systems biology approaches will enhance our ability to study virus-induced perturbations on cellular networks.
Next-generation sequencing technology will help define the genome and transcriptome for animals more comprehensively than traditional approaches, and enrich the data available for integrated approaches by providing greater information on novel splice variants, sRNAs and novel mutations.
Better computational methods for data integration is required in order to address challenges of data analysis and integration.
Early predictions of disease susceptibility and treatment response based on biomarkers are a first step toward personalized medicine.
Acknowledgments
The authors thank Eric Lower for preparation and design of the original illustration.
Footnotes
Financial & competing interests disclosure
Research in the authors’ laboratory is supported by federal grants from the National Institute of Allergy and Infectious Diseases, NIH, Department of Health and Human Services under contract number HHSN272200800060C and Public Health Service grants R01AI022646, R01HL080621, R24RR016354, P30DA015625, P01AI058113 and P51RR000166 from the NIH, USA. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
Jennifer R Tisoncik, Email: tisoncik@u.washington.edu, University of Washington, Department of Microbiology, Seattle, WA 98195-8070, USA, Tel.: +1 206 732 6120, Fax: +1 206 732 6056.
Sarah E Belisle, Email: sbelisle@u.washington.edu, University of Washington, Department of Microbiology, Seattle, WA 98195-8070, USA, Tel.: +1 206 732 6119, Fax: +1 206 732 6056.
Deborah L Diamond, Email: ddiamond@u.washington.edu, University of Washington, Department of Microbiology, Seattle, WA 98195-8070, USA, Tel.: +1 206 732 6047, Fax: +1 206 732 6056.
Marcus J Korth, Email: korth@u.washington.edu, University of Washington, Department of Microbiology, Seattle, WA 98195-8070, USA, Tel.: +1 206 732 6154, Fax: +1 206 732 6154.
Michael G Katze, Email: honey@u.washingon.edu, University of Washington, Department of Microbiology and Washington National Primate Research Center, Box 358070, Seattle, WA 98195-8070, USA, Tel.: +1 206 732 6135, Fax: +1 206 732 6056.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
- 1.Johnson NP, Mueller J. Updating the accounts: global mortality of the 1918–1920 “Spanish” influenza pandemic. Bull Hist Med. 2002;76(1):105–115. doi: 10.1353/bhm.2002.0022. [DOI] [PubMed] [Google Scholar]
- 2.Murray CJ, Lopez AD, Chin B, Feehan D, Hill KH. Estimation of potential global pandemic influenza mortality on the basis of vital registry data from the 1918–1920 pandemic: a quantitative analysis. Lancet. 2006;368(9554):2211–2218. doi: 10.1016/S0140-6736(06)69895-4. [DOI] [PubMed] [Google Scholar]
- 3.Tumpey TM, Basler CF, Aguilar PV, et al. Characterization of the reconstructed 1918 Spanish influenza pandemic virus. Science. 2005;310(5745):77–80. doi: 10.1126/science.1119392. [DOI] [PubMed] [Google Scholar]
- 4.Kash JC, Tumpey TM, Proll SC, et al. Genomic analysis of increased host immune and cell death responses induced by 1918 influenza virus. Nature. 2006;443(7111):578–581. doi: 10.1038/nature05181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kobasa D, Jones SM, Shinya K, et al. Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature. 2007;445(7125):319–323. doi: 10.1038/nature05495. [DOI] [PubMed] [Google Scholar]
- 6.Baas T, Baskin CR, Diamond DL, et al. Integrated molecular signature of disease: analysis of influenza virus-infected macaques through functional genomics and proteomics. J Virol. 2006;80(21):10813–10828. doi: 10.1128/JVI.00851-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolff GL, Kodell RL, Moore SR, Cooney CA. Maternal epigenetics and methyl supplements affect agouti gene expression in Avy/a mice. FASEB J. 1998;12(11):949–957. [PubMed] [Google Scholar]
- 8 ▪.Chesler EJ, Miller DR, Branstetter LR, et al. The Collaborative Cross at Oak Ridge National Laboratory: developing a powerful resource for systems genetics. Mamm Genome. 2008;19(6):382–389. doi: 10.1007/s00335-008-9135-8. Reports on the ongoing Collaborative Cross project initiated in 2005, which has bred a large population of genetically diverse mouse strains designed to support systems genetics analysis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Churchill GA, Airey DC, Allayee H, et al. The Collaborative Cross, a community resource for the genetic analysis of complex traits. Nat Genet. 2004;36(11):1133–1137. doi: 10.1038/ng1104-1133. [DOI] [PubMed] [Google Scholar]
- 10.Dolan MJ, Kulkarni H, Camargo JF, et al. CCL3L1 and CCR5 influence cell-mediated immunity and affect HIV–AIDS pathogenesis via viral entry-independent mechanisms. Nat Immunol. 2007;8(12):1324–1336. doi: 10.1038/ni1521. [DOI] [PubMed] [Google Scholar]
- 11.Fellay J, Shianna KV, Ge D, et al. A whole-genome association study of major determinants for host control of HIV-1. Science. 2007;317(5840):944–947. doi: 10.1126/science.1143767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Limou S, Le Clerc S, Coulonges C, et al. Genomewide association study of an AIDS-nonprogression cohort emphasizes the role played by HLA genes (ANRS Genomewide Association Study 02) J Infect Dis. 2009;199(3):419–426. doi: 10.1086/596067. [DOI] [PubMed] [Google Scholar]
- 13 ▪.Ahuja SK, Kulkarni H, Catano G, et al. CCL3L1–CCR5 genotype influences durability of immune recovery during antiretroviral therapy of HIV-1-infected individuals. Nat Med. 2008;14(4):413–420. doi: 10.1038/nm1741. Demonstrates that CCL3L1–CCR5 genetic variation influences reconstitution of CD4+ T cells in HIV patients receiving highly active antiretroviral therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kulkarni H, Agan BK, Marconi VC, et al. CCL3L1–CCR5 genotype improves the assessment of AIDS risk in HIV-1-infected individuals. PLoS One. 2008;3(9):e3165. doi: 10.1371/journal.pone.0003165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Catano G, Kulkarni H, He W, et al. HIV-1 disease-influencing effects associated with ZNRD1, HCP5 and HLA-C alleles are attributable mainly to either HLA-A10 or HLA-B*57 alleles. PLoS One. 2008;3(11):e3636. doi: 10.1371/journal.pone.0003636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shao W, Lazaryan A, Dorak MT, et al. Cohort- and time-specific associations of CTLA4 genotypes with HIV-1 disease progression. AIDS. 2006;20(12):1583–1590. doi: 10.1097/01.aids.0000238403.08497.3f. [DOI] [PubMed] [Google Scholar]
- 17.Le QM, Kiso M, Someya K, et al. Avian flu: isolation of drug-resistant H5N1 virus. Nature. 2005;437(7062):1108. doi: 10.1038/4371108a. [DOI] [PubMed] [Google Scholar]
- 18.Dharan NJ, Gubareva LV, Meyer JJ, et al. Infections with oseltamivir-resistant influenza A (H1N1) virus in the United States. JAMA. 2009;301(10):1034–1041. doi: 10.1001/jama.2009.294. [DOI] [PubMed] [Google Scholar]
- 19.McHutchison JG, Everson GT, Gordon SC, et al. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N Engl J Med. 2009;360(18):1827–1838. doi: 10.1056/NEJMoa0806104. [DOI] [PubMed] [Google Scholar]
- 20.Hezode C, Forestier N, Dusheiko G, et al. Telaprevir and peginterferon with or without ribavirin for chronic HCV infection. N Engl J Med. 2009;360(18):1839–1850. doi: 10.1056/NEJMoa0807650. [DOI] [PubMed] [Google Scholar]
- 21.Agbottah E, de La Fuente C, Nekhai S, et al. Antiviral activity of CYC202 in HIV-1-infected cells. J Biol Chem. 2005;280(4):3029–3042. doi: 10.1074/jbc.M406435200. [DOI] [PubMed] [Google Scholar]
- 22.Salerno D, Hasham MG, Marshall R, et al. Direct inhibition of CDK9 blocks HIV-1 replication without preventing T-cell activation in primary human peripheral blood lymphocytes. Gene. 2007;405(1–2):65–78. doi: 10.1016/j.gene.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan EY, Qian WJ, Diamond DL, et al. Quantitative analysis of human immunodeficiency virus type 1-infected CD4+ cell proteome: dysregulated cell cycle progression and nuclear transport coincide with robust virus production. J Virol. 2007;81(14):7571–7583. doi: 10.1128/JVI.00288-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24 ▪.Chan EY, Sutton JN, Jacobs JM, et al. Dynamic host energetics and cytoskeletal proteomes in human immunodeficiency virus type 1-infected human primary CD4 cells: analysis by multiplexed label-free mass spectrometry. J Virol. 2009;83(18):9283–9295. doi: 10.1128/JVI.00814-09. Demonstrates the use of proteomics in an HIV-1 infection system to profile virus-induced cellular perturbations during the course of infection and the effects of protein abundance changes in the presence of reverse-transcriptase inhibitor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinson JA, Tenorio AR, Montoya CJ, et al. Impact of class A, B and C CpG-oligodeoxynucleotides on in vitro activation of innate immune cells in human immunodeficiency virus-1 infected individuals. Immunology. 2007;120(4):526–535. doi: 10.1111/j.1365-2567.2007.02530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gulick RM, Lalezari J, Goodrich J, et al. Maraviroc for previously treated patients with R5 HIV-1 infection. N Engl J Med. 2008;359(14):1429–1441. doi: 10.1056/NEJMoa0803152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan SL, Ganji G, Paeper B, Proll S, Katze MG. Systems biology and the host response to viral infection. Nat Biotechnol. 2007;25(12):1383–1389. doi: 10.1038/nbt1207-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brass AL, Dykxhoorn DM, Benita Y, et al. Identification of host proteins required for HIV infection through a functional genomic screen. Science. 2008;319(5865):921–926. doi: 10.1126/science.1152725. [DOI] [PubMed] [Google Scholar]
- 29.Hao L, Sakurai A, Watanabe T, et al. Drosophila RNAi screen identifies host genes important for influenza virus replication. Nature. 2008;454(7206):890–893. doi: 10.1038/nature07151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aman MJ, Kinch MS, Warfield K, et al. Development of a broad-spectrum antiviral with activity against Ebola virus. Antiviral Res. 2009;83(3):245–251. doi: 10.1016/j.antiviral.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 31.Diaz L, Cassellaa J, Bonaviaa A, et al. Recruitment of the TSG101/ESCRT-I machinery in host cells by influenza virus: implications for broad-spectrum therapy. Presented at: 22nd International Conference on Antiviral Research; Miami, FL, USA. 3–7 May 2009. [Google Scholar]
- 32 ▪.Baskin CR, Bielefeldt-Ohmann H, Garcia-Sastre A, et al. Functional genomic and serological analysis of the protective immune response resulting from vaccination of macaques with an NS1-truncated influenza virus. J Virol. 2007;81(21):11817–11827. doi: 10.1128/JVI.00590-07. Showcases the use of transcriptional profiling integrated with proteomic analysis to investigate innate immune responses in a nonhuman primate model of influenza infection with the potential to identify immune correlates of vaccination. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buchbinder SP, Mehrotra DV, Duerr A, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372(9653):1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lederer S, Favre D, Walters KA, et al. Transcriptional profiling in pathogenic and nonpathogenic SIV infections reveals significant distinctions in kinetics and tissue compartmentalization. PLoS Pathog. 2009;5(2):e1000296. doi: 10.1371/journal.ppat.1000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35 ▪.Favre D, Lederer S, Kanwar B, et al. Critical loss of the balance between Th17 and T regulatory cell populations in pathogenic SIV infection. PLoS Pathog. 2009;5(2):e1000295. doi: 10.1371/journal.ppat.1000295. Functional genomics suggests a mechanism differentiating pathogenic from nonpathogenic SIV infection in nonhuman primates. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Querec TD, Akondy RS, Lee EK, et al. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat Immunol. 2009;10(1):116–125. doi: 10.1038/ni.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gaucher D, Therrien R, Kettaf N, et al. Yellow fever vaccine induces integrated multilineage and polyfunctional immune responses. J Exp Med. 2008;205(13):3119–3131. doi: 10.1084/jem.20082292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghosh Z, Mallick B, Chakrabarti J. Cellular versus viral microRNAs in host–virus interaction. Nucleic Acids Res. 2009;37(4):1035–1048. doi: 10.1093/nar/gkn1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu LF, Liston A. MicroRNA in the immune system, microRNA as an immune system. Immunology. 2009;127(3):291–298. doi: 10.1111/j.1365-2567.2009.03092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Munger J, Bajad SU, Coller HA, Shenk T, Rabinowitz JD. Dynamics of the cellular metabolome during human cytomegalovirus infection. PLoS Pathog. 2006;2(12):e132. doi: 10.1371/journal.ppat.0020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Munger J, Bennett BD, Parikh A, et al. Systems-level metabolic flux profiling identifies fatty acid synthesis as a target for antiviral therapy. Nat Biotechnol. 2008;26(10):1179–1186. doi: 10.1038/nbt.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ringrose JH, Jeeninga RE, Berkhout B, Speijer D. Proteomic studies reveal coordinated changes in T-cell expression patterns upon infection with human immunodeficiency virus type 1. J Virol. 2008;82(9):4320–4330. doi: 10.1128/JVI.01819-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van ‘t Wout AB, Swain JV, Schindler M, et al. Nef induces multiple genes involved in cholesterol synthesis and uptake in human immunodeficiency virus type 1-infected T cells. J Virol. 2005;79(15):10053–10058. doi: 10.1128/JVI.79.15.10053-10058.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walters KA, Syder AJ, Lederer SL, et al. Genomic analysis reveals a potential role for cell cycle perturbation in HCV-mediated apoptosis of cultured hepatocytes. PLoS Pathog. 2009;5(1):e1000269. doi: 10.1371/journal.ppat.1000269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wikoff WR, Pendyala G, Siuzdak G, Fox HS. Metabolomic analysis of the cerebrospinal fluid reveals changes in phospholipase expression in the CNS of SIV-infected macaques. J Clin Invest. 2008;118(7):2661–2669. doi: 10.1172/JCI34138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wikoff WR, Kalisak E, Trauger S, Manchester M, Siuzdak G. Response and recovery in the plasma metabolome tracks the acute LCMV-induced immune response. J Proteome Res. 2009;8(7):3578–3587. doi: 10.1021/pr900275p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peng X, Li Y, Walters KA, et al. Computational identification of hepatitis C virus associated microRNA–mRNA regulatory modules in human livers. BMC Genomics. 2009;10:373. doi: 10.1186/1471-2164-10-373. [DOI] [PMC free article] [PubMed] [Google Scholar]