Figure 7. Exposure of normal skin to acid pH is followed by a decrease in basal serine protease (SP) activity and kallikrein (klk) 5 and 7 activation.
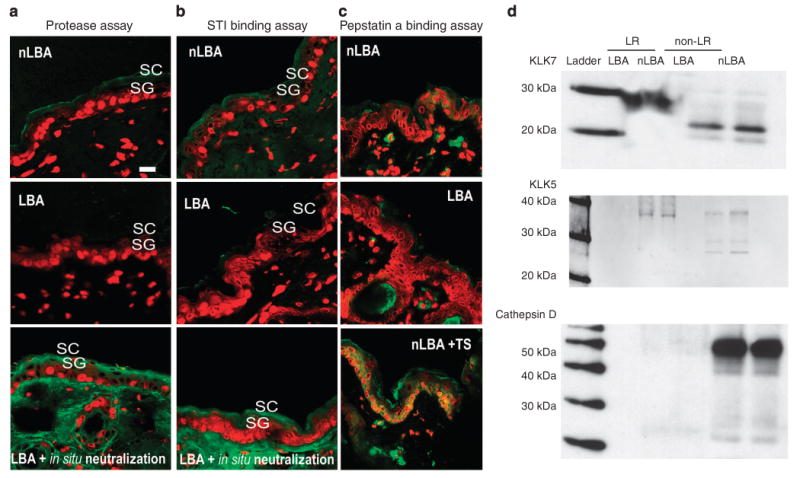
(a and b) In situ zymography for serine protease (SP) and soybean trypsin inhibitor (STI) binding assay 3 hours after PHA treatment shows a decrease in SP activity on SC acidification sites: LBA- vs nLBA-treated sites. The decrease in SP activities is not due to a decrease in enzyme mass as in situ neutralization overrides LBA effect by reactivating SP. Yet fluorescent pepstatin A binding assay (c) was carried out on sections from LBA, nLBA-treated normal skin or nLBA-treated tape-stripped (TS) skin to assess cathepsin D activity. Unlike barrier abrogation (TS + nLBA), SC acidification alone does suffice to increase enzyme activity, suggesting a secondary trigger besides pH for cathepsin D activation. (d) Western immunoblotting for klk7 and 5 shows decreased immunostaining for the active forms for both proteases (23 and 31 kDa, respectively) in the non-lipid raft (LR) fraction. Note the restriction of pro-klk5 and 7 to the LR domains. A similar pattern of pro-cathespin D (52 kDa) expression with a virtually absent active cathepsin D on both LBA- and nLBA-treated sites was found. Unlike klk5 and 7, pro-cathespin D is restricted to non-LR domains.
