Abstract
More than 80% of addicted individuals fail to seek treatment, which might reflect impairments in recognition of severity of disorder. Considered by some as intentional deception, such `denial' might instead reflect dysfunction of brain networks subserving insight and selfawareness. Here we review the scant literature on insight in addiction and integrate this perspective with the role of: (i) the insula in interoception, self-awareness and drug craving; (ii) the anterior cingulate in behavioral monitoring and response selection (relevant to disadvantageous choices in addiction); (iii) the dorsal striatum in automatic habit formation; and (iv) drug related stimuli that predict emotional behavior in addicted individuals, even without conscious awareness. We discuss implications for clinical treatment including the design of interventions to improve insight into illness severity in addiction.
INTRODUCTION
Based on the latest report from the US Department of Health and Human Services, only 4.5% of the 21.1 million persons classified in 2006 as needing, but not receiving, substance use treatment, reported a perceived need for therapy1. Therefore, one of the greatest challenges in drug addiction treatment is that the individuals who require treatment do not even recognize the need for therapeutic help. This treatment resistance may reflect in part the failure of society to recognize addiction as a disease and the blame and repudiation placed on the afflicted individuals. We propose here that this impairment may also reflect dysfunction of the neural circuits underlying interoception, self-awareness, and appropriate social, emotional and cognitive responses. Understanding these neuronal circuits could improve therapeutic strategies for treating addiction.
Interoception, self-awareness, and consciousness are interrelated concepts, collectively used to illustrate the ability to recognize and describe one's own (and others') behaviors, cognitions and mental states (see Box 1: What is insight?). Dysfunctional insight characterizes various neuropsychiatric disorders, spanning classical neurological insults (e.g., causing visual neglect or anosognosia for hemiplegia) to classical psychiatric disorders (e.g., schizophrenia, mania and other mood disorders), as recently reviewed2. In brief, impaired awareness in these disorders can take the form of failure to recognize an illness, denial of illness, compromised control of action and unawareness of the patient's social incompetence. Although seemingly disparate, the signs and symptoms of impaired awareness in these disorders have been organized into coherent theoretical frameworks. These models primarily highlight internal representations (of the actual, desired and predicted states of our own body and external world)3 that possibly utilize the dynamic interactions of specialized component processes via a distributed neural network4. Damage to specific sets of neural circuits may interrupt the internal signals that indicate a problem. Thus, the absence of information about the left side of one's body is no more worrisome than lack of visual information from behind one's head – no impairment is registered because no such input is expected4. An intact interpretive process continues to supply explanations that seem self-evident, even when exceedingly wrong4 (e.g., I am not using my left hand, not because it is paralyzed but because someone is preventing me from using it).
BOX 1: WHAT IS INSIGHT?
The terms insight, interoception, and awareness are often used interchangeably; however, interoception is not synonymous with subjective awareness nor is it clear whether conscious perception of interoceptive signals is sufficient or necessary for insightful action. In general, these terms may be distinguished along the following three lines: (1) sensorimotor: feeling a particular state, separate from having explicit knowledge of this state; (2) emotional: might indicate that a person comprehends the implications of a situation, also separate from factual knowledge; and (3) cognitive: the conscious process of thinking, separate from the recognition or achievement of a goal-state. Importantly, each of these terms has a potentially unique contribution to drug addiction. For example, interoception is defined as the sense of the physiological condition of the entire body or as a generalized homeostatic sensory capacity that underpins a conscious representation of how we feel21; two of its characteristics are important for addiction. First, interoceptive feelings are associated with intense affective and motivational components. This is not unlike drug cravings, which tend to be linked with overwhelming approach behaviors in heavy users. Second, the motivational evaluation of bodily signals normally depends on the homeostatic state of the individual, as exemplified by the contrasting feelings of reward or punishment produced by a simple cool object at different core body temperatures, yet in addicted individuals such signals may be misinterpreted (e.g., the drug is always wanted as there is no satiety for the drug). Related is the concept that the internal state itself can influence the degree to which individuals are able to accurately report their interoceptive state. For example, individuals with high levels of anhedonia relative to those with no anhedonia were less responsive to emotion-eliciting images across measures of heart rate, affective self-rated mood, and facial expressions69. In contrast, subjects with high emotional reactivity show high trait anxiety and a high degree of interoceptive awareness based on the heart-beat detection task70, 71. Similarly, individuals with panic attacks report more cardiac sensations and more frequent aversive interoceptive events than healthy control subjects72. Parallel studies in addiction are yet to be performed. Finally, one has to consider the correlation between insight/awareness with general intellectual functioning73–76 and study the extent to which cognitive impairment (which has now been reliably documented in drug addiction77, 78) may increase the risk for impaired self-awareness.
In the current opinion article, we argue that as a cognitive disorder5, drug addiction may share with these neuropsychiatric disorders similar abnormalities in self-awareness and behavioral control that can be attributed to an underlying neural dysfunction. These commonalities could include a dissociation between self-report and behavior. Thus, forced-choice behavior (e.g., choice between two alternatives) may indicate non-random behavior while the spontaneous attempt to explain this behavior may be compromised or lacking. Specifically, similar to blind-sightedness, where patients report they cannot see the visual cues that actually guide their behavior6, one could conceptualize drug addiction as a compromised ability to recognize external and internal drug-related cues. Such attenuated awareness of these cues may lead to the false belief that one is in control over drug taking behavior. An associated lack of recognition that one is afflicted by a disease or an underestimation of the severity of illness in drug addiction may drive these individuals to use drugs excessively, where control of use becomes exceedingly dysregulated.
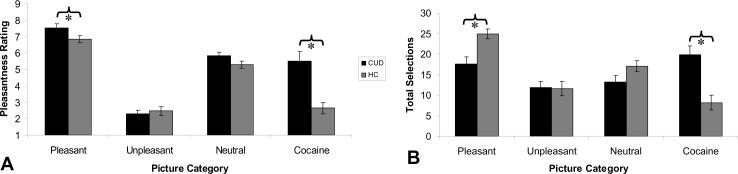
Consistent with this view, there is some appreciation of altered awareness as part of the diagnosis of drug dependence in the Diagnostic and Statistical Manual of Mental Disorders, the main consensus criteria for psychiatric diagnosis, where emphasis is placed on continued drug use despite knowledge of negative consequences. Indeed, only a minority of heavy drinkers define their own drinking as problematic even in the face of acknowledged negative consequences7. It is also well known that self-reported (conscious) craving is a poor predictor of relapse8. We recently reported a discordance between self-reported motivation and goal-driven behavior in cocaine addicted individuals9 as illustrated by the forced-choice results depicted in Figure 110. This discordance is mirrored by brain-behavior dissociations in tasks of reward processing11, behavioral monitoring and emotional suppression12. This internal discordance (self-report vs. behavior or brain-behavior) can be validated by a discrepancy between the patients' self-report and informants' reports (e.g., by a family member or a treatment-provider)13; correlations with neuropsychological performance14 support the notion that neurocognitive dysfunction underlies such compromised self-awareness, frequently mislabeled as “denial” (which assumes a priori knowledge, and intent to negate or minimize, the severity of symptoms).
Figure 1.
Self-reported pleasantness ratings (A) and objective choice behavior (total picture viewing selections) on an implicit choice task (B) for each of four picture types (pleasant, unpleasant, neutral, and cocaine) for individuals with cocaine use disorders (CUD; N=20), as compared to healthy comparison subjects (HC; N=20). For both (A) and (B), error bars represent standard error of the mean. * p<0.05. Results show that although CUD rated the pleasant pictures as more pleasant than the cocaine pictures (A), providing higher pleasantness ratings than the controls, the CUD's objective choice behavior did not show this effect (here controls chose to view the pleasant pictures more than the CUD, who also did not show enhanced pleasant picture over cocaine picture choice) (B). Adapted with permission from10.
Although drug addiction may also share with the other neuropsychiatric disorders a resistance to evidence-based or cognitively-driven changes in self-awareness4, self-awareness enhancements may improve treatment outcome possibly through impact on select neuropsychological functions (e.g., enhancing accuracy of self-report15, motivation or sense of agency16). For example, higher risk awareness (of the link between cigarette smoking and heart disease) was associated with a self-reported desire to reduce smoking in a very large sample of young adults17. In addition, better awareness of severity of alcohol use predicted actual abstinence for up to one year after treatment in 117 male alcoholics18. Nevertheless, self-awareness enhancements may also increase the salience of negative affect15, which may lead to increased drug use to alleviate the associated negative affective state. Thus, modulating self-awareness should be well monitored and expertly supervised, especially in addicted individuals with comorbid psychiatric disorders. An example for the interaction between baseline self-awareness and alcohol use in response to negative reinforcement is provided in Box 2: Self-awareness and alcohol.
BOX 2: SELF-AWARENESS AND ALCOHOL.
Impairments in social cognition including facial affect perception, emotional prosody, theory of mind, empathy, and related skills (e.g., humor processing) have been documented in alcoholics [review79]. For example, alcoholics overestimate the intensity of emotions even for neutral faces, with a bias towards overestimation of anger, fear and, in general, negative emotions. This facial affect perception impairment, related to the rostral anterior cingulate cortex and executive dysfunction, may lead to enhanced social conflict, stress and relapse; it may also be ameliorated by abstinence79. Importantly, these social cognition impairments may be related to self-awareness compromises (e.g., through mechanisms shared by awareness to self and others). In a related conceptualization (reviewed in80, 81), alcohol reduces the individual's level of self-awareness by inhibiting higher order cognitive processes related to (attending, encoding or sensitivity to) self-relevant information, a sufficient condition to induce and sustain further alcohol consumption. Specifically, this model proposes that alcohol can decrease negative self-evaluation (e.g., self-criticism), providing psychological relief but also decreasing the correspondence between behavior with external and internal standards of appropriate conduct. In support of this model, a series of studies80, 81 showed that (a) alcohol consumption as compared with a placebo control reduced relative usage or recall of self-referenced statements and first-person pronouns (measures of self-evaluation); and (b) high private self-consciousness (a tendency for an individual to direct attention inward towards thoughts, feelings, and behaviors, conceptually similar to interoception) was associated with more alcohol use (measured by actual drinking in male social drinkers, relapse after three months of detoxification in alcoholics, or self-reported use in adolescents) under negative feedback (failure feedback for performance on a previous task, negative life events, or academic achievement)80, 81. High private self-consciousness was also associated with higher urge to drink during alcohol cue reactivity paradigms in alcoholics82. Although moderating factors may include negative mood, reactivity or social context83, these studies suggest that individuals who are high on interoception or self-awareness may be susceptible to consuming alcohol following personal failure (or other negative reinforcement) where alcohol is used to avoid the ensuing unpleasant self-aware state. These results also suggest that in selected individuals, personal success (or other positive reinforcement) may decrease alcohol (and possibly other drug) consumption. Importantly, these studies suggest that insight manipulations need to take into account the context (e.g., negative or positive) and the baseline propensity of individuals for self-awareness.
Given that self-awareness and interoception seem crucial to understanding drug addiction and its treatment, here we review their putative underlying neural circuits. Abnormalities in the insula and medial regions of the prefrontal cortex (which include the anterior cingulate and mesial orbitofrontal cortices), and in subcortical regions (including the striatum), have been highlighted when comparing drug addicted individuals to neurological patients with focal brain damage19. These same corticolimbic brain regions have been associated with interoception and behavioral control, and with interrelated functions (habit formation and valuation), as reviewed below. These considerations expand the conceptualization of addiction beyond its association with the reward circuit, neurocognitive impairments in response inhibition and salience attribution5 and neuroadaptations in memory circuits20, to include compromised interoception, self-awareness and insight into illness.
INTEROCEPTION AND BEHAVIORAL CONTROL: UNIQUE AND CONJOINT ROLES FOR THE INSULA AND ANTERIOR CINGULATE CORTICES
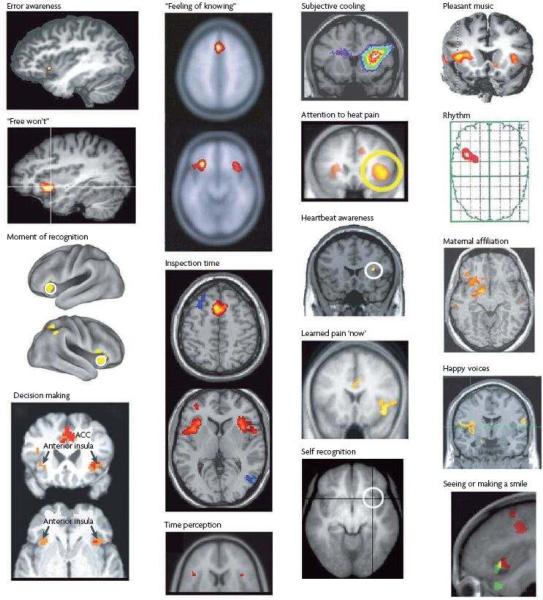
The posterior insula in primates contains interoceptive representation of the physiological condition of the body21. This activity is integrated in the middle insula with salient activity from all sensory and sensorimotor pathways, from homeostatic forebrain structures (amygdala, hypothalamus), and from systems concerned with reward and salience processing (ventral striatum)21. The anterior insula of humans further integrates emotionally salient activity from other forebrain regions, including the orbitofrontal, dorsolateral prefrontal, and anterior cingulate cortices, for its re-representation within a rich interoceptive foundation21. Indeed, the anterior insula is uniquely activated during all subjective feelings from the body and feelings of emotion (Figure 2), and recent evidence strongly supports the concept that the anterior insula engenders the representation of the sentient self that underlies phenomenal awareness21. For example, the size of the insular cortex has been directly related to interoceptive and emotional awareness, to empathic feelings, and to cooperative social behavior22–24. A lesion of the right insula can produce anosognosia for hemiplegia or hemianesthesia25, 26.
Figure 2.
A summary of imaging results showing activation of the anterior insular cortex (AIC) during particular tasks and emotions. The two right-hand columns contain images from different studies that found predominantly unilateral activation; images in the two left-hand columns show mostly bilateral activation. Stimuli that activate the right AIC are generally arousing to the body (for example, pain). The left AIC is activated mainly by positive and affiliative emotional feelings. ACC, anterior cingulate cortex. First published in21.
The anterior cingulate cortex, which comprises several parts that are unique in terms of cytoarchitecture and neurotransmitter receptor organization27, is also part of this network implicated in conscious and subjective experiences (such as pain28). For example, anterior cingulate cortex hypoactivations (posterior, rostral, pre- and sub-genual sectors) have been reported in states of compromised consciousness (vegetative state, minimally conscious state, seizures, sleep)28. Damage to the ventromedial sector of the prefrontal cortex and adjacent anterior cingulate cortex, is associated with unawareness of the patient's social incompetence29.
The anterior cingulate and bilateral anterior insula are conjointly active during perceptual awareness of visual or auditory stimuli, and they are parametrically correlated with the “feeling-of-knowing” and with performance on a visual inspection time task (that is directly related to psychometric intelligence)21. Degenerating (Von Economo) neurons in both cortices have been correlated with loss of emotional self-awareness and self-conscious behaviors in fronto-temporal dementia30. Together, the anterior insula and the anterior cingulate cortices can be viewed as complementary advanced limbic sensory (insula, awareness) and motor (cingulate, agency) regions that are conjointly activated during almost all human emotions and behaviors, analogous to the primary somatosensory and motor regions of the Rolandic cortex21, 31.
Distinctive roles for the anterior insula and anterior cingulate cortices have also been suggested. For example, contrasting errors (on a hybrid Stroop and Go/No-Go task) of which participants were aware with those of which they were unaware revealed sizeable fronto-parietal activation that encompassed the left insula; conversely, the rostral and dorsal anterior cingulate cortex was equally active for both error types32. Similar neuroanatomical dissociations have been observed using an anti-saccade task33. Further functional dissociations between these cortices on cognitive control tasks identify the dorsal anterior cingulate cortex with behavioral processes that can be disentangled from error processes and the insula with error awareness. For example, a large portion of the posterior medial frontal cortex including the dorsal anterior cingulate cortex may adjust behavioral agency in the context of response selection, central to implementing control (including cognitive control34) over one's behavior. Magno and colleagues have shown a selective role for the rostral and dorsal anterior cingulate cortex on tasks in which one can intervene in ongoing behavior in order either to avoid a likely error or to obtain an especially high reward35, 36. This online adjustment of behavior by the cingulate is consistent with a recent preclinical lesion study37, and may occur without subjective awareness. This contrasts with the observed pattern for the bilateral insula that was selectively active on error trials35, 36. Because the insula has also been implicated in post-error slowing38 and post-error strategy change39, it may have a role in the behavioral changes that are perhaps indicative of a conscious recognition of the need to overcome established response patterns and develop new behavioral strategies.
Taken together, the dorsal anterior cingulate may be central to on-line cognitive control and consequential for the decision-making of drug users through adjustments in response options (including risky options); one's meta-cognitive awareness of that behavior may involve the insula as a likely structure for interoceptive awareness that accompanies errors (or drug use effects) or the explicit motivation promoting drug use (e.g., conscious drug urges) as described next.
INSULA AND ANTERIOR CINGULATE ROLES IN DRUG ADDICTION
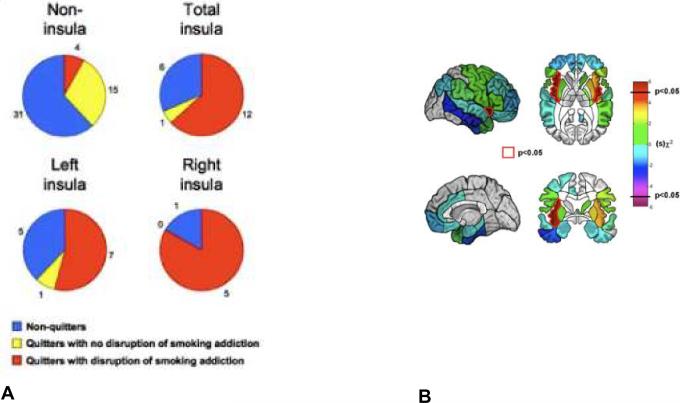
A recent study explored the role of insula damage in addiction40. In a retrospective design assessing changes in cigarette smoking after brain damage, 19 smokers who sustained damage in the insula were compared with 50 smokers who sustained damage in other brain areas. Results revealed that smokers with brain damage involving the insula were significantly (more than 100 times) more likely than smokers with brain damage not involving the insula to undergo a “disruption of smoking addiction”, characterized by the ability to quit smoking easily, immediately, without relapse and without a persistence of the urge to smoke (Figure 3). In some cases, this disruption was so profound as to lead some patients to proclaim that their “body forgot the urge to smoke”. This finding is consistent with the crucial role of the middle insula in cravings for food, cocaine and cigarettes41–43 as reported by neuroimaging studies. This finding was corroborated by a study in rats44 that showed that disruption of insula activity (by focal injection of lidocaine) stopped an already established addiction to amphetamine, thus suggesting that the insula is a key region for urges derived not only from smoking, but perhaps from other abused substances such as amphetamine.
Figure 3.
Patients who quit smoking after lesion onset and patients who underwent a disruption of smoking addiction after lesion onset and whole-brain region-by-region logistic regression analysis. Pie charts (A) illustrate the proportion of patients in each anatomical group who fell into each of the behavioral categories. Regions (B) for which there was a statistically significant association between a lesion and a disruption of smoking addiction (P < 0.05, uncorrected) are highlighted in red. The insula was the only region on either side of the brain where a lesion was significantly associated with a disruption of smoking addiction. Adapted with permission from40.
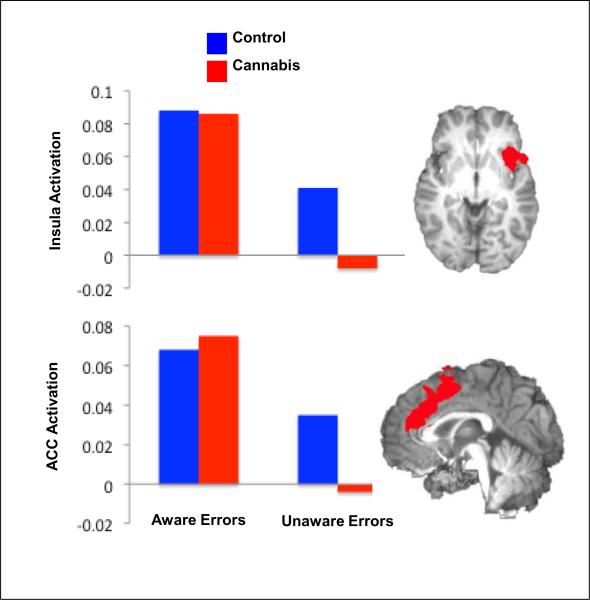
Reduced activity in the anterior cingulate cortex, mostly on selective attention and inhibitory control tasks, is a common observation in drug users, reported for cocaine, heroin, alcohol, cannabis and other drugs (review45). A recent study reported blunted rostral and dorsal anterior cingulate (and insula) response to predict diminished awareness of performance errors in cannabis users (in controls only the insula separated aware vs. unaware errors and the potential differences in the role of the cingulate as a function of drug addiction remains to be studied)46 (Figure 4); these findings are consistent with prior behavioral and fMRI studies in cocaine users45.
Figure 4.
Regions of brain activity differentiating Aware from Unaware Errors in 16 healthy control subjects and 17 young cannabis users. Bar graphs represent mean BOLD % signal change (relative to baseline) for each group during Aware and Unaware errors. Adapted with permission from46.
Note that anterior cingulate cortex hypoactivation levels cannot be attributed to task difficulty or disengagement but that, nevertheless, emotional salience modulates this region's responses in proportion to drug use severity47. Specifically, rostroventral anterior cingulate cortex (Brodmann Area 10, 11, implicated in successful emotion suppression) hypoactivations to an emotionally salient task were associated with task-induced craving suppression while caudaldorsal anterior cingulate cortex (Brodmann Area 32) hypoactivations were associated with higher frequency of current cocaine use in individuals with current cocaine use disorders47. Anterior cingulate cortex activation levels have also been shown to be predictive of successful treatment outcome in alcoholics and methamphetamine users48, 49 and can be improved by acute cocaine administration50 (this restorative effect is likely to be dose-specific51) in cocaine users.
In summary, although the exact roles and interactions of the anterior insula and anterior cingulate in driving awareness and control in addiction remain to be determined, it is most likely that abnormalities in the insula contribute to intense drug cravings and compromised insight and awareness of disease severity, while abnormalities in the cingulate contribute to the disadvantageous decision-making that precipitate relapse.
OTHER REGIONS AND PROCESSES IMPLICATED IN AWARENESS IN DRUG ADDICTION
An influential theoretical account has posited that the `switch' from voluntary drug use to habitual and progressively compulsive drug use represents a transition at the neural level from prefrontal cortical to striatal control over drug-seeking and drug-taking behaviors, as well as a progression from ventral to more dorsal domains of the striatum, mediated at least in part by its stratified dopaminergic innervation (see review52). Specifically, lateral parts of the dorsal striatum – the site at which dopamine release is increased during habitual drug seeking and where dopamine receptor antagonist infusions impair this behavior – dominate the stimulus-response instrumental process in which drug seeking behavior becomes a response habit triggered and maintained by drug-associated stimuli; disconnecting the ventral-dorsal striatal loops greatly and selectively decreases such habitual cocaine seeking in rats52. Similarly in humans, the presentation of drug cues to cocaine addicted individuals both induce drug craving and activate the dorsal striatum, which shows reductions in dopamine D2 receptor availability in abstinent alcoholics, cocaine, heroin and methamphetamine addicted individuals20. This theoretical account thus characterizes compulsive drug seeking as a maladaptive stimulus-response habit in which the ultimate goal of the behavior has been devalued, perhaps through tolerance to the rewarding effects of the drug52. Importantly, the compromised insight into severity of addiction in drug addicted individuals could at least in part be driven by this switch to an automatic and habitual system, which may operate outside awareness (automatic processes require less attention, effortful control and conscious awareness53). It is also possible that compromised awareness enhances the influence of automatized action schemata54 leading to uncontrollable drug-seeking behaviors (especially during high-risk situations)55. Finally, it is conceivable that to be subjectively perceived or to drive behavior, these internal drive states (`must do!'52 parallel to the subjective state of excessive `wanting' embodied in the incentive salience sensitization view of addiction56) need to be processed by the anterior insula.
The involvement of other regions in compromised awareness in addiction remains to be explored. For example, we would predict a central role for the orbitofrontal cortex, where function (glucose metabolism as possibly modulated by striatal dopamine receptor availability) and structure (grey matter volume) are reduced in drug addicted individuals (review in5). Given the role of the orbitofrontal cortex in expectation57 and accurate self-evaluations58, in addition to its well-established role in reversal of stimulus-reinforcement associations (review in59), one could predict its interpretive role (e.g., alternative explanations for or minimization of severity of addiction) in insight and awareness.
DRUG-RELATED STIMULI OUTSIDE AWARENESS ACTIVATE BRAIN MOTIVATIONAL CIRCUITS AND PREDICT FUTURE POSITIVE AFFECT TO VISIBLE DRUG CUES
Conscious drug desire is a hallmark feature of the addictions60. Afflicted individuals can sometimes identify stimuli that preceded the desire state: “I was OK until I suddenly saw an old broken crack (cocaine) pipe in the gutter; then the craving hit me …”. Often, however, users are unaware of triggers: “Doc, I really don't know what happened. One minute I was OK, and the next second the craving hit me, and I was off on a mission to get the drug…”. “Volcanic” craving that erupts suddenly into consciousness – and feels almost impossible to control – is a common terror for patients, and a difficult target for therapists. Identifying patients' conscious triggers for drug craving, in an attempt to manage them, has become part of many mainstream treatments. However, one implication of diminished self-awareness in addiction is that behavioral control may be undermined by motivational processes of which the patient is largely unaware. Stated differently (alluded to in the section above), what if the powerful drug motivational machinery is indeed set in motion outside awareness, where it is relatively impervious to therapist and patient efforts to gain insight or awareness about its triggers?
The notion that powerful motivational states can occur outside awareness was popularized by Freud at the start of the 20th century, but was difficult to test directly. However, “fast” imaging technologies are now capable of capturing the brain response to very brief visual stimuli – presented in a way that prevents conscious recognition. A recent study used “fast” event-related fMRI in 21 cocaine patients to test whether 33 msec “unseen” cocaine (and sexual) cues activated the limbic reward circuitry (this duration was intentionally chosen to keep the cues below the threshold for conscious recognition)61. Results showed that both the cocaine and sexual “unseen” cues activated the ventral striatum/pallidum, amygdala, anterior insula, and caudolateral orbitofrontal cortex, paralleling prior studies of reward circuitry in humans and in animals61 [note that although the anterior insula was activated, the 33 msec individual cues remained “unseen”. Therefore, the anterior insula activation in this paradigm may reflect the interoceptive state (feelings) generated by the unseen cues that, it would appear, can occur without explicit awareness (knowledge) of those cues, see Box 1]. Importantly, ventral pallidum/amygdala activation to the “unseen” cocaine cues predicted future positive affect to visible versions of these same cues (two days later). This correlation demonstrated the functional significance of the “unseen” cues, consistent with recent reports showing that appetitive signals (e.g., for money62, a tasty juice63) outside of conscious attention can influence ongoing (e.g., grip force62) or subsequent (e.g., seat choice63) motivated behavior.
TREATMENT IMPLICATIONS
Motivational interviewing is a frequent intervention in drug abuse treatment used to enhance the readiness for change and to maintain that change. However, cue-triggered appetitive motivation that begins almost instantly, outside awareness, may not be amenable to such an insight-oriented approach and indeed deterioration over time in the effectiveness of insight-oriented psychotherapies in addicted individuals has been documented64. Alternative interventions may include cognitive training directed at the neuropsychological mechanisms that may underlie impaired self-awareness. For example, a recent study suggested that attentional training can reduce attention bias to drug-related stimuli in drug users (drinkers) as associated with post-training reductions in alcohol consumption that were maintained three months after training65. Such cognitive biases to drug-related cues may also be reduced by pharmacological interventions aimed at bolstering supportive cognitive functions (e.g., serotonergic, dopaminergic and noradrenergic modulation of reversal learning, set-shifting, inhibitory control or impulsivity; reviewed in66). Because more severe cognitive impairment is associated with worse treatment retention rates in this population67, the use of pharmacological or other cognitive remediation techniques may decrease treatment drop-out and subsequently enhance clinical outcome. Together, interventions based on understanding the dysfunctional brain circuitry as reviewed here could complement, and potentially enhance, insight-oriented or cognitive-behavioral68 approaches, increasing self-awareness (so the drug addicted individual can recognize and explicitly acknowledge the need to change or detect circumstances when relapse likelihood is increased) and providing more comprehensive relapse protection for addicted individuals.
SUMMARY AND FUTURE DIRECTIONS
Here we put forth the argument that insight and awareness are compromised in drug addicted individuals as possibly related to an underlying neural dysfunction in the brain regions that modulate interoception, behavioral monitoring, self-evaluation, and habit formation. However, direct empirical evidence for such impairment in drug addiction is scarce. Our major aim is therefore to call for future scientific exploration of the neural basis of insight and awareness in addiction. Tractable and quantifiable neurocognitive assays of awareness need to be developed for this particular purpose. These tools need not rely on self-report exclusively. Instead, direct measures of behavior and emotion need to be used (e.g., tests of implicit/automatic processing including stem-completion, cue-reactivity, semantic priming, and other subliminal perception or implicit learning paradigms, also tasks of choice behavior and decision-making). Of particular value is the development of tools that could be used simultaneously with functional neuroimaging or psychophysiological brain recordings. Test-retest studies are essential because the level of awareness and insight may vary according to the different stages within the addiction cycle (including intoxication, drug expectation/preparation, bingeing, withdrawal, relapse5). For example, one could postulate that under emotionally stressful situations (e.g., intense craving) self-awareness may be particularly impacted. Similarly to the increased vulnerability of some individuals to become addicted (and to relapse, e.g., high impulsive individuals52), individual differences in compromised awareness also remain to be tested. Such a fine-tuned analysis could offer opportunities for tailored interventions that would specifically target the most vulnerable individuals at their most vulnerable times within the addiction cycle. In general, better understanding of insight, awareness and interoception in addiction may bridge the gap between self-report (e.g., craving) and objective behavioral (cue-reactivity) measures to enhance treatment outcome in drug addiction.
Acknowledgements/funding
The contribution of Steven J. Grant, Division of Clinical Neuroscience and Behavioral Research, National Institute on Drug Abuse, for reviewing and editing this manuscript is gratefully acknowledged. This manuscript is based on a symposium on `Functional Neuroimaging Evidence for a Brain Network Underlying Impaired Insight Into Illness in Drug Addiction' chaired by RZG and co-chaired by SJG at the 2008 annual meeting of the Society for Neuroscience, Washington DC. The preparation of this manuscript was supported by grants from the National Institute on Drug Abuse (to RZG: 1R01DA023579 and R21DA02062; to AB: R01DA023051; to HG: DA014100) and the Barrow Neurological Foundation (to ADC).
Footnotes
Conflicts of Interest Statement: Rita Goldstein consulted for Medical Directions, Inc. She has also received honoraria for speaking at seminars on Law and Neuroscience, co-sponsored by the Federal Judicial Center, Gruter Institute for Law and Behavioral Research, New York University, and the Catherine T. and John D. MacArthur Foundation Law & Neuroscience Project. Martin Paulus has consulted for Sepracor, Roche, and GSK and has received grant support from these pharmaceutical companies.
Notice: This manuscript has been authored by Brookhaven Science Associates, LLC under Contract No. DE-AC02-98CHI-886 with the U.S. Department of Energy. The United States Government retains, and the publisher, by accepting the article for publication, acknowledges, a world-wide license to publish or reproduce the published form of this manuscript, or allow others to do so, for the United States Government purposes.
REFERENCES (MAIN TEXT)
- 1.SAMHSA . Results from the 2006 National Survey on Drug Use and Health: National Findings. 2007. (Office of Applied Studies, NSDUH Series H-32, DHHS Publication No. SMA 07-4293) [Google Scholar]
- 2.Orfei MD, et al. Unawareness of illness in neuropsychiatric disorders: phenomenological certainty versus etiopathogenic vagueness. Neuroscientist. 2008;14:203–222. doi: 10.1177/1073858407309995. [DOI] [PubMed] [Google Scholar]
- 3.Blakemore SJ, et al. Abnormalities in the awareness of action. Trends Cogn Sci. 2002;6:237–242. doi: 10.1016/s1364-6613(02)01907-1. [DOI] [PubMed] [Google Scholar]
- 4.Cooney JW, Gazzaniga MS. Neurological disorders and the structure of human consciousness. Trends Cogn Sci. 2003;7:161–165. doi: 10.1016/s1364-6613(03)00058-5. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. American Journal of Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clifford CW, et al. Getting technical about awareness. Trends Cogn Sci. 2008;12:54–58. doi: 10.1016/j.tics.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 7.McLennan JD, et al. Adolescents' insight in heavy drinking. J Adolesc Health. 1998;22:409–416. doi: 10.1016/s1054-139x(97)00201-2. [DOI] [PubMed] [Google Scholar]
- 8.Miller NS, Gold MS. Dissociation of “conscious desire” (craving) from and relapse in alcohol and cocaine dependence. Ann Clin Psychiatry. 1994;6:99–106. doi: 10.3109/10401239409148988. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein RZ, et al. Is decreased prefrontal cortical sensitivity to monetary reward associated with impaired motivation and self-control in cocaine addiction? Am J Psychiatry. 2007;164:43–51. doi: 10.1176/appi.ajp.164.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moeller SJ, et al. Enhanced Choice for Viewing Cocaine Pictures in Cocaine Addiction. Biol Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldstein RZ, et al. Compromised sensitivity to monetary reward in current cocaine users: an ERP study. Psychophysiology. 2008;45:705–713. doi: 10.1111/j.1469-8986.2008.00670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldstein RZ, et al. Anterior cingulate cortex hypoactivations to an emotionally salient task in cocaine addiction. Proc Natl Acad Sci U S A. 2009;106:9453–9458. doi: 10.1073/pnas.0900491106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verdejo-Garcia A, Perez-Garcia M. Substance abusers' self-awareness of the neurobehavioral consequences of addiction. Psychiatry Res. 2008;158:172–180. doi: 10.1016/j.psychres.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Rinn W, et al. Addiction denial and cognitive dysfunction: a preliminary investigation. J Neuropsychiatry Clin Neurosci. 2002;14:52–57. doi: 10.1176/jnp.14.1.52. [DOI] [PubMed] [Google Scholar]
- 15.Gibbons FX, et al. Self-awareness and self-confrontation: effects of self-focused attention on members of a clinical population. J Pers Soc Psychol. 1985;48:662–675. doi: 10.1037//0022-3514.48.3.662. [DOI] [PubMed] [Google Scholar]
- 16.Haggard P. Conscious intention and motor cognition. Trends Cogn Sci. 2005;9:290–295. doi: 10.1016/j.tics.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 17.Steptoe A, et al. Tobacco smoking in young adults from 21 European countries: association with attitudes and risk awareness. Addiction. 1995;90:571–582. doi: 10.1046/j.1360-0443.1995.90457111.x. [DOI] [PubMed] [Google Scholar]
- 18.Kim JS, et al. The role of alcoholics' insight in abstinence from alcohol in male Korean alcohol dependents. J Korean Med Sci. 2007;22:132–137. doi: 10.3346/jkms.2007.22.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- 20.Volkow ND, et al. The addicted human brain: insights from imaging studies. J Clin Invest. 2003;111:1444–1451. doi: 10.1172/JCI18533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 22.Critchley HD, et al. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- 23.Silani G, et al. Levels of emotional awareness and autism: an fMRI study. Soc Neurosci. 2008;3:97–112. doi: 10.1080/17470910701577020. [DOI] [PubMed] [Google Scholar]
- 24.King-Casas B, et al. The rupture and repair of cooperation in borderline personality disorder. Science. 2008;321:806–810. doi: 10.1126/science.1156902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karnath HO, et al. Awareness of the functioning of one's own limbs mediated by the insular cortex? J Neurosci. 2005;25:7134–7138. doi: 10.1523/JNEUROSCI.1590-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spinazzola L, et al. Modular structure of awareness for sensorimotor disorders: evidence from anosognosia for hemiplegia and anosognosia for hemianaesthesia. Neuropsychologia. 2008;46:915–926. doi: 10.1016/j.neuropsychologia.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 27.Palomero-Gallagher N, et al. Cytology and receptor architecture of human anterior cingulate cortex. J Comp Neurol. 2008;508:906–926. doi: 10.1002/cne.21684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laureys S. The neural correlate of (un)awareness: lessons from the vegetative state. Trends Cogn Sci. 2005;9:556–559. doi: 10.1016/j.tics.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 29.Bechara A. Disturbances of emotion regulation after focal brain lesions. Int Rev Neurobiol. 2004;62:159–193. doi: 10.1016/S0074-7742(04)62006-X. [DOI] [PubMed] [Google Scholar]
- 30.Seeley WW, et al. Divergent social functioning in behavioral variant frontotemporal dementia and Alzheimer disease: reciprocal networks and neuronal evolution. Alzheimer Dis Assoc Disord. 2007;21:S50–57. doi: 10.1097/WAD.0b013e31815c0f14. [DOI] [PubMed] [Google Scholar]
- 31.Heimer L, Van Hoesen GW. The limbic lobe and its output channels: implications for emotional functions and adaptive behavior. Neurosci Biobehav Rev. 2006;30:126–147. doi: 10.1016/j.neubiorev.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 32.Hester R, et al. Neural mechanisms involved in error processing: a comparison of errors made with and without awareness. Neuroimage. 2005;27:602–608. doi: 10.1016/j.neuroimage.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 33.Klein TA, et al. Neural correlates of error awareness. Neuroimage. 2007;34:1774–1781. doi: 10.1016/j.neuroimage.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 34.Ridderinkhof KR, et al. The role of the medial frontal cortex in cognitive control. Science (New York, N.Y. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- 35.Magno E, et al. The anterior cingulate and error avoidance. J Neurosci. 2006;26:4769–4773. doi: 10.1523/JNEUROSCI.0369-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Magno E, et al. A brain network for reward-related performance evaluation. Journal of Cognitive Neuroscience. in press. [Google Scholar]
- 37.Modirrousta M, Fellows LK. Dorsal medial prefrontal cortex plays a necessary role in rapid error prediction in humans. J Neurosci. 2008;28:14000–14005. doi: 10.1523/JNEUROSCI.4450-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li CS, et al. Neural correlates of post-error slowing during a stop signal task: a functional magnetic resonance imaging study. J Cogn Neurosci. 2008;20:1021–1029. doi: 10.1162/jocn.2008.20071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paulus MP, et al. Reduced behavioral and neural activation in stimulant users to different error rates during decision making. Biol Psychiatry. 2008;63:1054–1060. doi: 10.1016/j.biopsych.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naqvi NH, et al. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bonson KR, et al. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002;26:376–386. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- 42.Pelchat ML, et al. Images of desire: food-craving activation during fMRI. Neuroimage. 2004;23:1486–1493. doi: 10.1016/j.neuroimage.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 43.Wang Z, et al. Neural substrates of abstinence-induced cigarette cravings in chronic smokers. J Neurosci. 2007;27:14035–14040. doi: 10.1523/JNEUROSCI.2966-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Contreras M, et al. Inactivation of the interoceptive insula disrupts drug craving and malaise induced by lithium. Science. 2007;318:655–658. doi: 10.1126/science.1145590. [DOI] [PubMed] [Google Scholar]
- 45.Garavan H, Stout JC. Neurocognitive insights into substance abuse. Trends Cogn Sci. 2005;9:195–201. doi: 10.1016/j.tics.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 46.Hester R, et al. Impaired error awareness and anterior cingulate cortex hypoactivity in chronic cannabis users. Neuropharmacology. 2009 doi: 10.1038/npp.2009.67. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goldstein RZ, et al. Anterior cingulate cortex hypoactivations to an emotionally salient task in cocaine addiction. Proc Natl Acad Sci U S A. 2009:1–6. doi: 10.1073/pnas.0900491106. Early Edition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grusser SM, et al. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology (Berl) 2004;175:296–302. doi: 10.1007/s00213-004-1828-4. [DOI] [PubMed] [Google Scholar]
- 49.Paulus MP, et al. Neural activation patterns of methamphetamine-dependent subjects during decision making predict relapse. Arch Gen Psychiatry. 2005;62:761–768. doi: 10.1001/archpsyc.62.7.761. [DOI] [PubMed] [Google Scholar]
- 50.Garavan H, et al. Acute effects of cocaine on the neurobiology of cognitive control. Philos Trans R Soc Lond B Biol Sci. 2008;363:3267–3276. doi: 10.1098/rstb.2008.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fillmore MT, et al. Acute effects of cocaine in two models of inhibitory control: implications of non-linear dose effects. Addiction. 2006;101:1323–1332. doi: 10.1111/j.1360-0443.2006.01522.x. [DOI] [PubMed] [Google Scholar]
- 52.Everitt BJ, et al. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 54.Tiffany ST. A cognitive model of drug urges and drug-use behavior: role of automatic and nonautomatic processes. Psychol Rev. 1990;97:147–168. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- 55.Rohsenow DJ, et al. Cue reactivity as a predictor of drinking among male alcoholics. J Consult Clin Psychol. 1994;62:620–626. doi: 10.1037//0022-006x.62.3.620. [DOI] [PubMed] [Google Scholar]
- 56.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 57.Volkow ND, et al. Expectation Enhances the Regional Brain Metabolic and the Reinforcing Effects of Stimulants in Cocaine Abusers. J Neurosci. 2003;23:11461–11468. doi: 10.1523/JNEUROSCI.23-36-11461.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beer JS. The default self: feeling good or being right? Trends Cogn Sci. 2007;11:187–189. doi: 10.1016/j.tics.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 59.Rolls ET. The orbitofrontal cortex and reward. Cereb Cortex. 2000;10:284–294. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- 60.Childress AR, et al. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Childress AR, et al. Prelude to passion: limbic activation by “unseen” drug and sexual cues. PLoS ONE. 2008;3:e1506. doi: 10.1371/journal.pone.0001506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pessiglione M, et al. How the brain translates money into force: a neuroimaging study of subliminal motivation. Science. 2007;316:904–906. doi: 10.1126/science.1140459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McCabe JA, et al. Appetitive and aversive taste conditioning in a computer game influences real-world decision making and subsequent brain activation. J Neurosci. 2009;29:1046–1051. doi: 10.1523/JNEUROSCI.3938-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McCambridge J, Strang J. Deterioration over time in effect of Motivational Interviewing in reducing drug consumption and related risk among young people. Addiction. 2005;100:470–478. doi: 10.1111/j.1360-0443.2005.01013.x. [DOI] [PubMed] [Google Scholar]
- 65.Fadardi JS, Cox WM. Reversing the sequence: reducing alcohol consumption by overcoming alcohol attentional bias. Drug Alcohol Depend. 2009;101:137–145. doi: 10.1016/j.drugalcdep.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 66.Vocci FJ. Cognitive remediation in the treatment of stimulant abuse disorders: a research agenda. Exp Clin Psychopharmacol. 2008;16:484–497. doi: 10.1037/a0014101. [DOI] [PubMed] [Google Scholar]
- 67.Aharonovich E, et al. Cognitive deficits predict low treatment retention in cocaine dependent patients. Drug Alcohol Depend. 2006;81:313–322. doi: 10.1016/j.drugalcdep.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 68.Carroll KM, et al. Enduring effects of a computer-assisted training program for cognitive behavioral therapy: a 6-month follow-up of CBT4CBT. Drug Alcohol Depend. 2009;100:178–181. doi: 10.1016/j.drugalcdep.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
BOX 1
- 69.Ferguson ML, Katkin ES. Visceral perception, anhedonia, and emotion. Biol Psychol. 1996;42:131–145. doi: 10.1016/0301-0511(95)05151-1. [DOI] [PubMed] [Google Scholar]
- 70.Pollatos O, et al. Interoceptive awareness, anxiety and cardiovascular reactivity to isometric exercise. Int J Psychophysiol. 2007;65:167–173. doi: 10.1016/j.ijpsycho.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 71.Pollatos O, et al. Interoceptive awareness mediates the relationship between anxiety and the intensity of unpleasant feelings. J Anxiety Disord. 2007;21:931–943. doi: 10.1016/j.janxdis.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 72.Vaitl D. Interoception. Biol Psychol. 1996;42:1–27. doi: 10.1016/0301-0511(95)05144-9. [DOI] [PubMed] [Google Scholar]
- 73.Grudnik JL, Kranzler JH. Meta-analysis of the relationship between intelligence and inspection time. Intelligence. 2001;29:523–535. [Google Scholar]
- 74.Cook ET, et al. The relations between emotional understanding, intellectual functioning, and disruptive behavior problems in elementary-school-aged children. J Abnorm Child Psychol. 1994;22:205–219. doi: 10.1007/BF02167900. [DOI] [PubMed] [Google Scholar]
- 75.Rabbitt P. Age, IQ and awareness, and recall of errors. Ergonomics. 1990;33:1291–1305. doi: 10.1080/00140139008925333. [DOI] [PubMed] [Google Scholar]
- 76.Frodi A, Smetana J. Abused, neglected, and nonmaltreated preschoolers' ability to discriminate emotions in others: the effects of IQ. Child Abuse Negl. 1984;8:459–465. doi: 10.1016/0145-2134(84)90027-9. [DOI] [PubMed] [Google Scholar]
- 77.Goldstein RZ, et al. Severity of neuropsychological impairment in cocaine and alcohol addiction: association with metabolism in the prefrontal cortex. Neuropsychologia. 2004;42:1447–1458. doi: 10.1016/j.neuropsychologia.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 78.Woicik PA, et al. The neuropsychology of cocaine addiction: recent cocaine use masks impairment. Neuropsychopharmacology. 2009;34:1112–1122. doi: 10.1038/npp.2008.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
BOX 2
- 79.Uekermann J, Daum I. Social cognition in alcoholism: a link to prefrontal cortex dysfunction? Addiction. 2008;103:726–735. doi: 10.1111/j.1360-0443.2008.02157.x. [DOI] [PubMed] [Google Scholar]
- 80.Hull JG, et al. Applications of the self-awareness model of alcohol consumption: predicting patterns of use and abuse. J Pers Soc Psychol. 1986;51:790–796. doi: 10.1037//0022-3514.51.4.790. [DOI] [PubMed] [Google Scholar]
- 81.Hull JG, Young RD. Self-consciousness, self-esteem, and success-failure as determinants of alcohol consumption in male social drinkers. J Pers Soc Psychol. 1983;44:1097–1109. doi: 10.1037//0022-3514.44.6.1097. [DOI] [PubMed] [Google Scholar]
- 82.Bradizza CM, et al. Alcohol cue reactivity and private self-consciousness among male alcoholics. Addict Behav. 1999;24:543–549. doi: 10.1016/s0306-4603(98)00093-8. [DOI] [PubMed] [Google Scholar]
- 83.Frankenstein W, Wilson GT. Alcohol's effects on self-awareness. Addict Behav. 1984;9:323–328. doi: 10.1016/0306-4603(84)90030-3. [DOI] [PubMed] [Google Scholar]