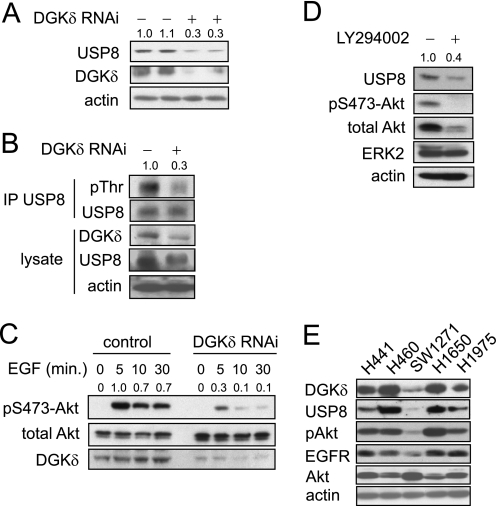
FIGURE 4.
DGKδ deficiency reduces the levels of USP8 and attenuates Akt phosphorylation. A, in two separate experiments, HeLa cells were transfected with scrambled or DGKδ siRNA duplexes and then USP8, DGKδ, and actin were detected in cell lysates by Western blotting. USP8 band densities (arbitrary units) are shown above the blot. B, HeLa cells were transfected with V5-USP8 and then with DGKδ or control siRNA duplexes. Cells grown in medium with serum were treated with calyculin A (100 nm) for 5 min, and then lysates were harvested and V5-USP8 was immunoprecipitated. The indicated proteins were detected by Western blotting, and pThr band densities (arbitrary units) are shown above the blot. C, HeLa cells were transfected with scrambled or DGKδ siRNA duplexes, starved for 16 h, and then treated with EGF (5 ng/ml) for the indicated times. Akt, p473-Akt, and DGKδ were detected in cell lysates by Western blotting. Band densities (arbitrary units) of phosphorylated Akt are shown above the blot. D, HeLa cells growing in medium with serum were treated with 20 μm LY294002 for 24 h, and then the levels of the indicated proteins were detected in cell lysates by Western blotting. USP8 band densities (arbitrary units) are shown above the blot. E, lung cancer cell lines (noted above each lane) were grown in complete medium and harvested, and then the indicated proteins were detected by Western blotting.