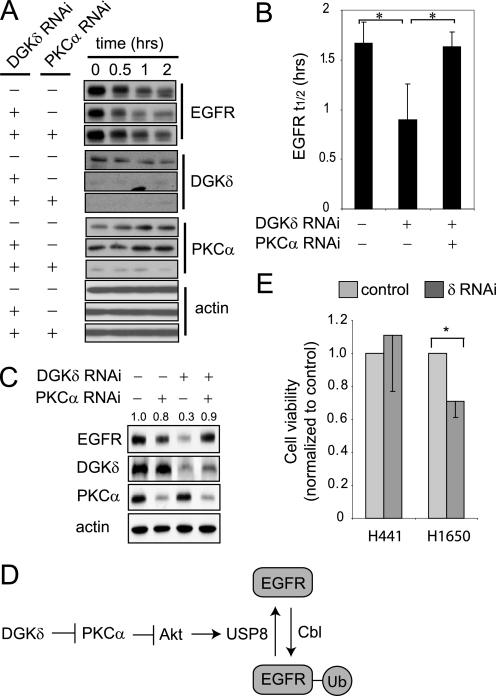
FIGURE 6.
Depleting PKCα rescues the EGFR defects caused by DGKδ deficiency. A, HeLa cells were transfected with scrambled or the indicated siRNA duplexes, starved, treated with cycloheximide (5 μm, 20 min), and then exposed to EGF (5 ng/ml for 0–2 h). EGFR, DGKδ, PKCα, and actin were detected in cell lysates by Western blotting. Samples are from the same blot, but are presented to allow comparison. B, the half-life (t½) of EGFR was determined from Western blots in three independent experiments. The asterisk indicates statistical significance (p < 0.05, n = 3). C, HeLa cells were transfected with scrambled, DGKδ, and/or PKCα siRNA duplexes, grown in medium with 10% serum, and then steady-state levels of EGFR, actin, DGKδ, and PKCα were detected by Western blotting. EGFR band densities (arbitrary units) are indicated above the blot. D, our data support a model where DGKδ and PKCα regulate the activity of Akt, which in turn modulates the levels of USP8 and consequently the amount of ubiquitin attached to EGFR. E, DGKδ was knocked down using RNAi in H441 lung cancer cells (which are resistant to EGFR inhibitors) or H1650 lung cancer cells (which are sensitive) grown in medium with 2% serum. At 72 h after knockdown, cell viability was examined. Viability (mean ± S.D.) is shown normalized to cells exposed to scrambled siRNA. The asterisk indicates statistical significance (p < 0.01, n = 4). Western blotting demonstrated similar knockdown of DGKδ and EGFR in both cell lines (not shown).