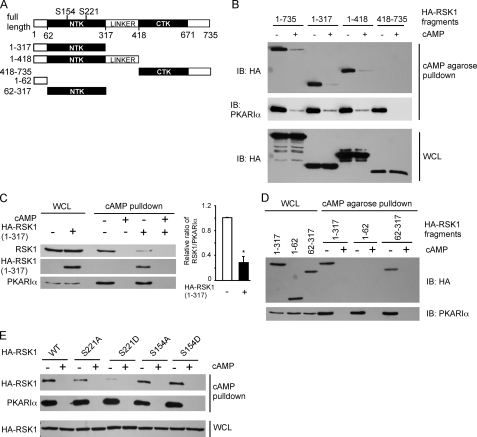
FIGURE 1.
RSK1, via its N-terminal kinase domain, interacts with PKARIα. A, shown is a schematic of rat RSK1 and HA-tagged RSK1 constructs used; numbers represent the amino acids in rat RSK1. B–E, shown is pulldown of HA-tagged RSK1 or its polypeptides with cAMP-agarose. HEK293T cells were transfected to express HA-tagged RSK1 or its polypeptides. After depriving of serum overnight, cells were lysed and incubated with cAMP-agarose to pull down PKARIα in the presence and absence of 50 mm cAMP as indicated. The proteins in the complex were monitored using anti-HA, anti-PKARIα, or anti-RSK1 antibodies. IB, immunoblot. B, PKARIα binds to RSK1 fragments containing the N-terminal part of RSK1. C, HA-RSK1-(1–317) competes with endogenous RSK1 for binding to PKARIα. The panel on the right shows quantification of band intensities of RSK1 as a ratio of band intensities of PKARIα (mean ± S.E.) from three experiments. *, p < 0.05 as compared with the control. D, the NTK of RSK1 binds to PKARIα. E, substitution of Ser-221 of RSK1 with a negatively charged residue abrogates the interaction between RSK1 and PKARIα. Representatives of three similar experiments are shown for all panels. WCL, whole cell lysates.