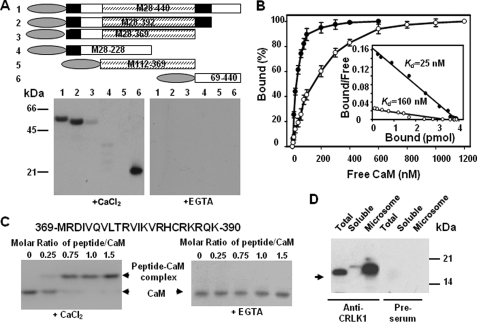
FIGURE 1.
CRLK1 is a Ca2+-dependent calmodulin-binding protein. A, CaM binds to CRLK1. CRLK1 deletion mutants (upper, domain structure is shown in the longest mutant, CaMBD is in a filled box, and kinase domain is in a hatched box) translationally fused with a His tag (oval box) were produced in E. coli. Purified recombinant proteins (2 μg) were subjected to SDS-PAGE and transferred onto a nitrocellulose membrane. The membrane was incubated with 50 nm 35S-PCM1 in a buffer containing either 2 mm EGTA or 0.1 mm CaCl2 (lower). B, saturation curve of 35S-CaM binding to purified M369-440 (solid circle) and M28–228 (open circle). Four pmol of recombinant proteins were spotted on a membrane and incubated with different amounts of 35S-PCM1. The amount of bound CaM at each point was represented as a percentage of the maximal binding. The average of three independent experiments was presented. The inset shows Scatchard plots of the binding data. C, gel mobility shift assay showing CaM binding to a synthetic peptide (amino acids 369–390 in CRLK1). D, CaM is co-immunoprecipitated with CRLK1 in planta. The total proteins or proteins from different fractions were prepared from wild-type plants treated at 4 °C for 4 h. Immunoprecipitation was carried out using anti-CRLK1 antibody. The immunoprecipitants were fractionated in SDS-PAGE and subjected to Western analysis. Calmodulin was detected using anti-calmodulin antibody.