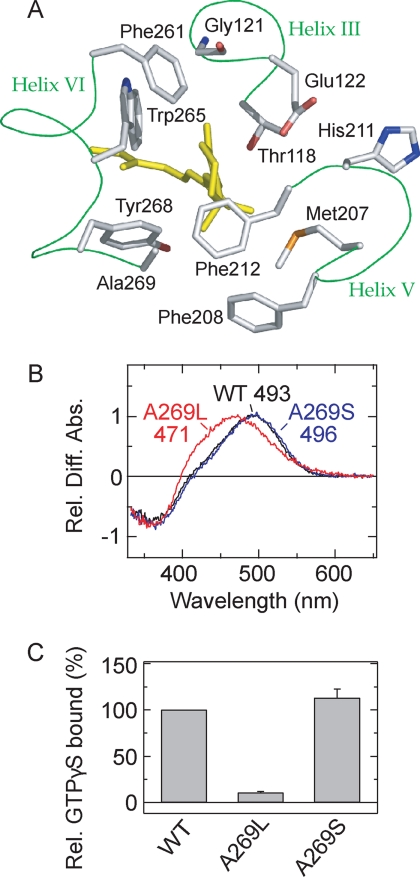
FIGURE 1.
Molecular properties of the active state of the parapinopsin Ala-269 mutant. A, 11-cis-retinal and amino acid residues around the β-ionone ring in the crystal structure of bovine rhodopsin (Protein Data Bank code 1U19) (10). 11-cis-Retinal (yellow) and amino acid residues within 4.5 Å of the β-ionone ring are shown. Phe-208 is also shown. Note that the backbone of the amino acid residues except Gly-121 is omitted. B, difference spectra of WT parapinopsin (black) and mutants A269L (red) and A269S (blue) in the membrane preparations. All difference absorbances at λmax of the active states were normalized to 1.0. The mutations introduced and the λmax values from the difference spectrum are also shown. Rel. Diff. Abs., relative difference absorbance. C, Gi activation rates of WT parapinopsin and mutants A269L and A269S. Each sample was purified with DM, and experiments were performed at 0 °C. Data are expressed as means ± S.E. of three separate experiments. Figs. 1A, 3A, 3B, and 6 were prepared using PyMOL.