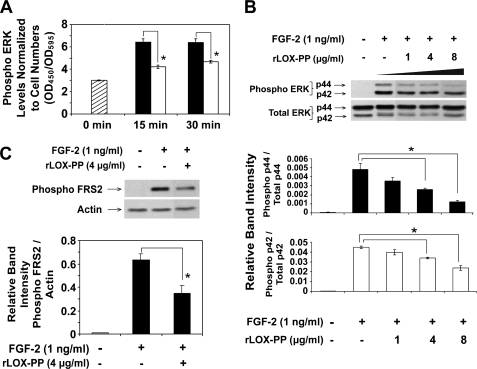
FIGURE 4.
rLOX-PP inhibits FGF-2-induced ERK1/2 and FRS2α phosphorylation determined by ELISA (A) and Western blotting (B). The effect of rLOX-PP on FGF-2-induced ERK1/2 activation was first analyzed using the FACETM (Active Motif). A, MC3T3-E1 cells were grown in 96-well plates for 24 h in medium containing 0.1% BSA. Cells were induced with FGF-2 (1 ng/ml) in the presence of 4 μg/ml rLOX-PP (white bars) or vehicle (black bars) for 0–30 min, after which the cells were fixed and later probed with primary antibodies against phospho-ERK1/2. Levels of phosphoprotein were determined colorimetrically (A450). Wells were subsequently stained with crystal violet to allow for normalization (A595). Bars indicate mean readings (A450/A595) for each group ± S.E. (n = 4, *, p < 0.05). In Western blotting experiments (B and C), MC3T3-E1 cells were plated in 6-well plates (8 × 104 cells/well), and after 24 h, cells were placed in medium containing 0.1% BSA for 24 h. Cells were then treated with FGF-2 (1 ng/ml) in the presence of 0–8 μg/ml rLOX-PP for 20 min (B) or 4 μg/ml rLOX-PP for 5 min (C). Cell lysates were collected in SDS-PAGE sample buffer and subjected to SDS-PAGE followed by Western blot analysis. Upper panels show representative blots probed with antibody specific for phosphorylated ERK1/2 (B) or phosphorylated FRS2α (Tyr-436) and for total ERK1/2 and β-actin for normalization (C). The lower panel shows mean values obtained from densitometric analysis of blots ± S.D. from one experiment (n = 3, *, p < 0.05). This experiment was performed twice with the same outcome.