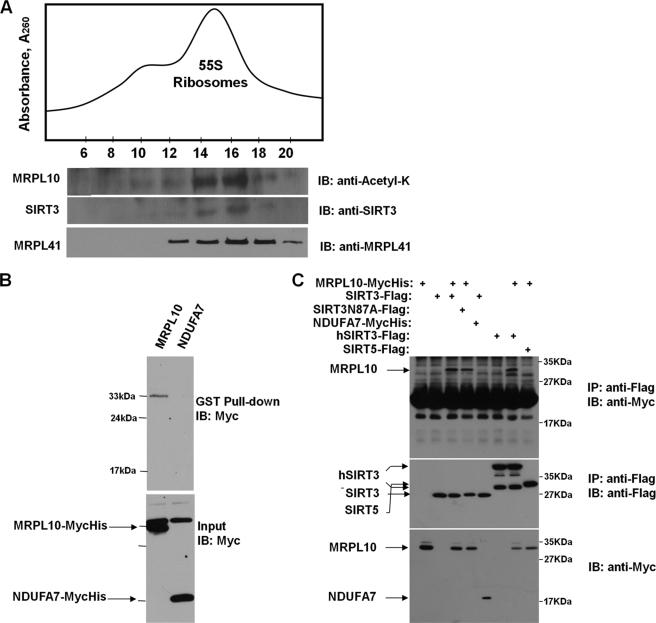
FIGURE 3.
Interactions between SIRT3 and mitochondrial 55 S ribosomes and MRPL10. A, crude mitochondrial ribosomes were loaded on to 10–30% linear sucrose gradients to sediment 55 S ribosomes. To demonstrate the co-sedimentation of SIRT3 with the 55 S ribosome, immunoblot (IB) analyses were performed with anti-N-acetyl lysine antibody detecting the acetylated MRPL10, as well as anti-SIRT3 anti-MRPL41 antibodies, after separating 30 μl of each fraction on 12% SDS-PAGE. B, lysates prepared from HEK293 cells transfected with MycHis-tagged MRPL10 or NDUFA7 were incubated with recombinant GST-SIRT3 fusion protein immobilized on the glutathione-conjugated agarose beads. The proteins associated with the beads were analyzed by immunoblotting using anti-Myc antibody. C, HEK293 cells were transfected with MycHis-tagged MRPL10 or NDUFA7 with or without FLAG-tagged murine SIRT3, SIRT3N87A mutant, human SIRT3, or murine SIRT5, as indicated. The cell lysates were immunoprecipitated (IP) with anti-FLAG-agarose beads and detected by immunoblotting with anti-Myc or anti-FLAG antibodies.