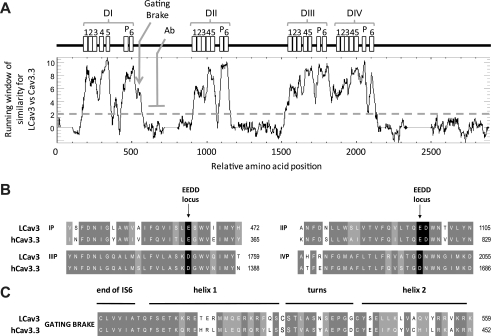
FIGURE 3.
Running window of similarity (A) and alignments (B and C) between amino acid sequences of distant T-type channel homologs (snail LCav3 and human Cav3.3) reveal that the invariant structures for T-type channels are harbored in six membrane-spanning segments in all four domains (I, II, III, and IV), including an ion conducting pore (S5-P-loop-S6) and voltage sensor (S1-S4). Illustrated is the position in the I-II linker where LCav3 polyclonal antibody (Ab) was generated in rabbits against a 200-amino acid peptide. B, shown is amino acid sequence alignment of the re-entrant P-loop located between S5 and S6 of each of the four domains illustrating the signature sequence (EEDD locus) that influences Ca2+ ion permeation and selectivity. The conserved aspartate residue (1097 in LCav3) in a position downstream of the selectivity filter glutamate residue is positioned to attract incoming Ca2+ ions to the pore.3 LCav3 contains a neutral isoleucine in the outer pore at position 468 where mammalian T-type channels have a negatively charged residue (Glu or Asp) that influence pore blocking drugs. C, alignment of the cytoplasmic gating brake in proximal I-II linker is shown. The gating brake is thought to prevent T-type channel gating at more hyperpolarized potentials.