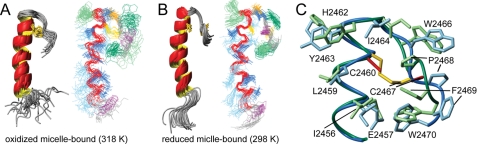
FIGURE 4.
The NMR structures of DPC-micelle bound oxidized (A) and reduced (B) y1fatc. Both plots show a ribbon representation of the superposition of the 20 lowest energy structures to the left and a line representation to the right. The side chains of the disulfide bonding forming cysteines (Cys-2460 and Cys-2467) are colored yellow. In the line representation the backbone of the α-helical stretch is depicted in red. Aromatic side chains are shown in light (F, H, Y) and dark (W) green, hydrophilic side chains in light blue, and those containing methyl groups in dark blue. Prolines are colored purple, and the conserved glycine (Gly-2465) that facilitates the chain reversal is in orange. C shows a superposition of the membrane-binding region of reduced (green, cysteine side chains in red) and oxidized (blue, cysteine side chains in yellow) y1fatc bound to DPC micelles. The side chains of Arg-2458 and Gln-2461 have been omitted for clarity. All structure images were made with the programs MolMol (55) and POV-Ray.