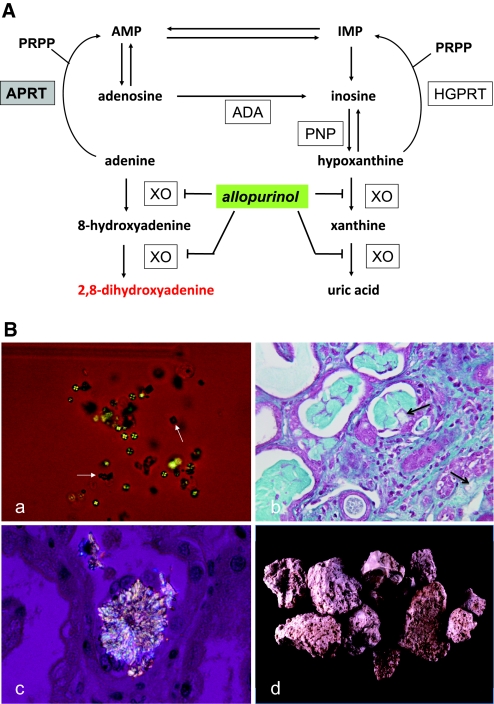
Figure 1.
APRT deficiency causes 2,8-DHA accumulation, leading to urolithiasis and crystalline nephropathy. (A) Metabolic pathways for the disposal of adenine in human show, in the absence of APRT activity, the alternative route of oxidation by xanthine oxydase (XO) to 2,8-DHA via the 8-hydroxy-intermediate in a manner analogous to the production of uric acid from hypoxanthine via xanthine. In human, adenine cannot be converted to adenosine as hypoxanthine to inosine by purine nucleoside phosphorylase (PNP); the only alternative pathway is oxidation. The site of inhibitory effect of allopurinol on 2,8-DHA synthesis is also indicated. ADA, adenosine deaminase; AMP, adenosine monophosphate; HGPRT, hypoxanthine-guanine phosphoribosyltransferase; IMP, inosine monophosphate; PRPP, 5-phosphoribosyl-1-pyrophosphate. (B) Morphologic features of 2,8-DHA crystals and stones. (a) Crystalluria study by polarized microscopy revealing typical 2,8-DHA crystals appearing round and reddish-brown with characteristic central Maltese cross pattern. Note the presence of few crystals of calcium oxalate dihydrate (white arrows) in addition to 2,8-DHA crystals. All crystalluria examined in our patients with APRT deficiency were positive for 2,8-DHA crystals. (b) Light microscopy study of kidney allograft biopsy, showing severe tubulointerstitial injury secondary to precipitation of crystals (arrows; Masson's Trichrome staining). (c) Kidney allograft biopsy examined by polarized microscopy showing precipitation of 2,8-DHA crystals within tubular lumen and in renal interstitium. (d) 2,8-DHA stones. Surface of stones are typically rough, humpy, soft, and friable. Color is reddish-brown turning grey when drying. Stone sections are disorganized with porosities and beige to brown color. Magnifications: ×200 in Ba; ×400 in B, b and c.