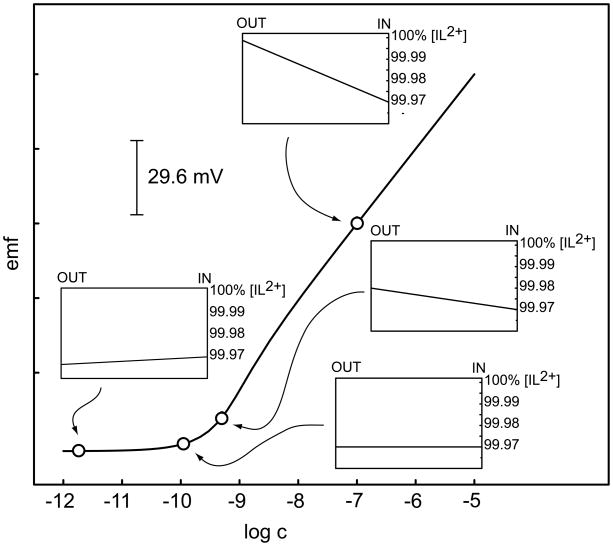
Fig. 2.
Calculated electrode response curve for a I2+-selective polymer membrane electrode at pH 7.0 with , RT = 5 mM, and q = 0.001 (cf. [2]). The static lower detection limit would be 10−17 M ((107)2 × 10−3). The inner solution is designed to induce a small net ion-exchange with interfering ions at the inner side of the membrane. At cI = 10−7 M, the concentration gradient within the membrane has no apparent effect on the observed potential since the sample is sufficiently concentrated. The maximum deviation from Nernst response is shown with sample cI = 10−9.3 M. Here, the inward flux depletes the sample phase boundary phase so that the concentration at the membrane surface is just half that of the sample bulk. At cI = 10−9.9 M, the same portion of I2+ is exchanged at both sides of the membrane so that no gradient is present and the emf response lies perfectly on the Nernst response curve. Further dilution of the sample leads to an outward flux of ions that completely dictates the response behavior of the electrode (see cI = 10−11.8 M).