Table 2.
Scope of the palladium-catalyzed 1,2-diarylation of styrene derivatives and 1,3-dienes with organostannanes.
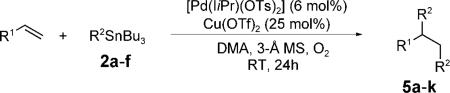 | ||||
|---|---|---|---|---|
| Entry | R1 | R2 | Product | Yield [%]a |
| 1 | p-MeC6H4 | Ph (2a) | 5a | 78 |
| 2 | p-MeC6H4 | p-FC6H4 (2b) | 5b | 64 |
| 3 | p-MeC6H4 | p-MeOC6H4 (2c) | 5c | 65b |
| 4 | p-MeC6H4 | m,m-(MeO)2C6H3 (2d) | 5d | 65 |
| 5 | p-MeC6H4 | p-CF3C6H4 (2e) | 5e | 68 |
| 6 | p-MeOC6H4 | Ph | 5f | 85 |
| 7 | o-MeC6H4 | Ph | 5g | 73 |
| 8 | o-MeOC6H4 | Ph | 5h | 73 |
| 9 | p-MeC6H4 |
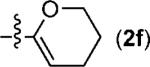
|
5i | 57 |
| 10c |
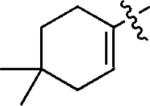
|
m,m-(MeO)2C6H3 | 5j | 55 |
| 11c | p-FC6H4 | 5k | 59 | |
| 12d |
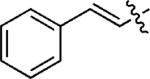
|
p-FC6H4 | 5l | 62 |
| 13d |
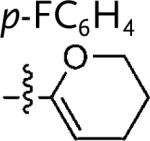
|
5m | 37 | |
Average yield of the isolated product in at least two experiments.
A 3:1 mixture of 1,2- and 1,1-diarylation products was formed.
The reaction was performed at 45 °C.
The reaction was performed at 40 °C.
