Table 2.
Hydrogenative coupling of vinyl azines 1b-1d to N-toluenesulfonyl aldimines 2f, 2i and 2k.
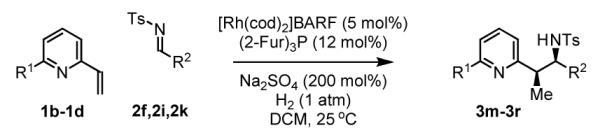 | ||
|---|---|---|
| 1b, R = Me | 1c, R = Ph | 1d, R = CH2OTBS |
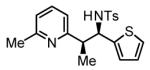 70% Yieldb 5:1 dr, 3m |
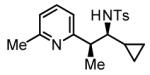 56% Yieldb 10:1 dr, 3n |
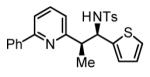 77% Yield 5:1 dr, 3o |
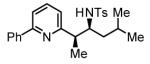 80% Yield 4:1 dr, 3p |
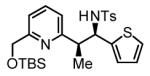 71% Yield 5:1 dr, 3q |
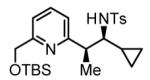 67% Yield 6:1 dr, 3r |
Cited yields are of isolated diastereomeric mixtures. Standard conditions employ 3 equiv. of 1b-1d and 1 equiv. of imines 2f, 2i and 2k. See Supporting Information for further details.
Reaction was performed using 7.5 mol% [Rh(cod)2]BARF and 18 mol% (2-Fur)3P.
