Abstract
OBJECTIVE
Sirtuin 1 (SIRT1) is implicated in the regulation of mitochondrial function, energy metabolism, and insulin sensitivity in rodents. No studies are available in humans to demonstrate that SIRT1 expression in insulin-sensitive tissues is associated with energy expenditure and insulin sensitivity.
RESEARCH DESIGN AND METHODS
Energy expenditure (EE), insulin sensitivity, and SIRT1 mRNA adipose tissue expression (n = 81) were measured by indirect calorimetry, hyperinsulinemic-euglycemic clamp, and quantitative RT-PCR in 247 nondiabetic offspring of type 2 diabetic patients.
RESULTS
High EE during the clamp (r = 0.375, P = 2.8 × 10−9) and high ΔEE (EE during the clamp − EE in the fasting state) (r = 0.602, P = 2.5 × 10−24) were associated with high insulin sensitivity. Adipose tissue SIRT1 mRNA expression was significantly associated with EE (r = 0.289, P = 0.010) and with insulin sensitivity (r = 0.334, P = 0.002) during hyperinsulinemic-euglycemic clamp. Furthermore, SIRT1 mRNA expression correlated significantly with the expression of several genes regulating mitochondrial function and energy metabolism (e.g., peroxisome proliferator–activated receptor γ coactivator-1β, estrogen-related receptor α, nuclear respiratory factor-1, and mitochondrial transcription factor A), and with several genes of the respiratory chain (e.g., including NADH dehydrogenase [ubiquinone] 1α subcomplex 2, cytochrome c, cytochrome c oxidase subunit IV, and ATP synthase).
CONCLUSIONS
Impaired stimulation of EE by insulin and low SIRT1 expression in insulin-sensitive tissues is likely to reflect impaired regulation of mitochondrial function associated with insulin resistance in humans.
Compromised mitochondrial function in skeletal muscle predisposes to insulin resistance and type 2 diabetes (1,2). In contrast, physical activity and weight loss in obese and sedentary subjects stimulate mitochondrial biogenesis and improve insulin sensitivity (3). Animal and human studies have shown that mitochondrial function is associated with insulin sensitivity, but the mechanisms explaining this association are largely unknown (4).
The mammalian sirtuins (SIRT1–SIRT7) are implicated in gene silencing, mitochondrial function, energy homeostasis, insulin sensitivity, and longevity (5). We previously demonstrated that treatment with SIRT1 activator, resveratrol, enhanced mitochondrial activity and protected mice from diet-induced obesity and insulin resistance (4). The effects of resveratrol were seen in both muscle and adipose tissue and resulted in an increase in mitochondrial function, which translated into an increase in energy expenditure (EE) and insulin sensitivity. Small molecule activators of SIRT1, that are structurally unrelated to resveratrol, have also been shown to improve insulin sensitivity, lower plasma glucose, and increase mitochondrial capacity (6). In many rodent models (4,7), the upregulation of the oxidative phosphorylation (OXPHOS) pathway is coordinated by peroxisome proliferator–activated receptor γ coactivator (PGC) 1α, which is a target of SIRT1 (8). Similarly, insulin resistance in human skeletal muscle has been associated with decreased mitochondrial oxidative capacity and ATP synthesis and decreased expression of genes that control mitochondrial activity, including PGC-1α (9–11).
The offspring of type 2 diabetic subjects are known to be insulin resistant, and they have defects in mitochondrial OXPHOS associated with increased intramyocellular lipid content (9). The association of EE and insulin sensitivity with SIRT1 and PGC-1α mRNA expression has not been previously investigated. Therefore, we studied here the association of EE, insulin sensitivity, and adipose tissue SIRT1 and PGC-1α mRNA expression in 247 nondiabetic offspring of subjects with type 2 diabetes.
RESEARCH DESIGN AND METHODS
The subjects were selected from an ongoing study and included healthy nondiabetic offspring of patients with type 2 diabetes, as previously described (12). The diabetic patients (probands) were randomly selected among type 2 diabetic subjects living in the region of the Kuopio University Hospital. Spouses of the probands had to have a normal glucose tolerance in an oral glucose tolerance test (OGTT). The study protocol was approved by the ethics committee of the University of Kuopio. All study subjects gave an informed consent. A total of 247 offspring (one to three from each family) were studied. Their mean ± SD age was 35.1 ± 6.3 years and BMI 26.3 ± 4.7 kg/m2.
Clinical and laboratory methods.
Height and weight were measured to the nearest 0.5 cm and 0.1 kg, respectively. BMI was calculated as weight (in kilograms) divided by square of height (in meters). On the first day, an OGTT was performed with 75 g of glucose. Subjects with normal glucose tolerance (n = 210), isolated impaired fasting glucose (n = 6), or impaired glucose tolerance (n = 31), defined on the basis of the World Health Organization criteria (13), were included in further studies. An intravenous glucose tolerance test (IVGTT) was performed to determine the first-phase insulin release after an overnight fast. After baseline blood collection, a bolus of glucose (300 mg/kg in a 50% solution) was given within 30 s into the antecubital vein. Samples for the measurement of blood glucose and plasma insulin (arterialized venous blood) were drawn at −5, 0, 2, 4, 6, 8, 10, 20, 30, 40, 50, and 60 min. After an IVGTT, the degree of insulin sensitivity was evaluated with the hyperinsulinemic-euglycemic clamp technique (insulin infusion rate of 40 mU · min−1 · m−2 body surface area) as previously described (14). Blood glucose was clamped at 5.0 mmol/l for the next 120 min by infusion of 20% glucose at various rates according to blood glucose measurements performed at 5-min intervals. The mean amount of glucose infused during the last 20 min of the clamp was used to calculate the rates of whole-body glucose uptake (WBGU) and divided with lean body mass (LBM) for statistical analyses. Nonoxidative glucose metabolism (per LBM) was calculated as the difference between the rates of WBGU/LBM and glucose oxidation (per LBM). Indirect calorimetry was performed with a computerized flow-through canopy gas analyzer system (Deltatrac; Datex) as previously described (14). The mean value of the data during the last 20 min of the clamp was used to calculate glucose and lipid oxidation. Protein, glucose, and lipid oxidation were calculated according to the method reported by Ferrannini (15). A computed tomography scan (Siemens Volume Zoom) at the level of fourth lumbal vertebra was performed to evaluate the amount of intra-abdominal and subcutaneous fat as previously described (14). Blood and plasma glucose were measured by the glucose oxidase method (Glucose and Lactate Analyzer 2300 Stat Plus; Yellow Springs Instruments), and plasma insulin was determined by radioimmunoassay (Phadeseph Insulin RIA 100, Pharmacia Diagnostics; 125J RIA Kit, Incstar, respectively). Serum free fatty acids (FFAs) were determined by an enzymatic method from Wako Chemicals. Nonprotein urinary nitrogen was measured by the automated Kjeldahl method as previously described (14). Plasma concentrations of tumor necrosis factor-α and cytokines (interleukin [IL]-1 receptor antagonist [IL-1RA], IL-6, IL-8, IL-10, IL-18, and interferon γ and serum levels of soluble adhesion molecules (intercellular adhesion molecule-1, vascular cell adhesion molecule-1, e-selectin, and p-selectin) were measured with high-sensitivity assay kits from R&D Systems. IL-8 was measured with a kit from Biosource International. High-sensitivity C-reactive protein (hs-CRP) was measured with an Immulite analyzer and a DPC hs-CRP assay.
Gene expression studies in offspring of type 2 diabetic parents.
Subcutaneous fat needle biopsies were taken after an overnight fast in a supine position at the level of the umbilicus during local anesthesia. Total RNA from adipose tissue (n = 81) was isolated with an RNeasy Lipid Tissue Mini kit (Qiagen) and treated with DNase (DNA-free; Ambion). Total RNA from skeletal muscle biopsies (n = 11) was isolated with QIAzol (Qiagen) and cleaned with an RNeasy Plus Micro Kit (Qiagen). RNA was transcribed to cDNA using random primers and a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Quantitative RT-PCRs were performed in a seven 500 Real-Time PCR System (Applied Biosystems) using 6 ng (RNA equivalents) of cDNA as template, gene-specific primers, and probes (Applied Biosystems) (information on primers and probes available upon request). Expression levels of different genes were normalized to large ribosomal protein P0 (adipose tissue: Hs99999902_ml; Applied Biosystems) or to β-actin (skeletal muscle: Hs99999903_m1; Applied Biosystems) using the standard curve method.
Sirt1 mRNA expression studies in mice.
Total RNA was isolated from adipose tissue samples of 5-month-old female transgenic (DBA/2 × BALB/c) mice using TRI reagent (Ambion), according to the manufacturer's guidelines. The resulting total RNA was subjected to DNase treatment using RNase-free DNase (Ambion). The purity of isolated RNA was measured by a NanoDrop spectrophotometer. A set concentration of RNA was reverse transcribed into cDNA, and quantitative PCR was performed on ABI Prism 7500 Sequence Detection System (Applied Biosystems). Inventoried Taqman primers for Sirt1 (Mm01168521_m1) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Mm99999915_g1) were purchased from Applied Biosystems. Sirt1 expression was normalized to the copy number of GAPDH.
Sirt1 protein expression studies in mice.
Adipose tissue samples were homogenized and proteins extracted using T-PER (no. 78510; Pierce) along with protease inhibitors and phosphatase inhibitors (Roche) from the same mice as in mRNA expression studies. After homogenization, tissue lysates were centrifuged for 30 min at high speed, and supernatant was collected and stored at −70°C until further analysis. Protein concentrations were measured with bicinchoninic acid protein assay kit (no. 23225; Pierce, Rockford, IL). A total of 20 μg/lane of protein samples containing NuPAGE LDS sample buffer (Invitrogen) and reducing agent were loaded into 4–12% NuPAGE Bis-Tris gels (Invitrogen) and were subjected to gel electrophoresis and transferred to polyvinylidene fluoride membranes (Amersham). For Sirt1 proteins, the membranes were blocked in Tris-buffered saline (TBS) with 3% milk and 0.05% Tween-20 for 1 h at room temperature, washed with TBS-0.05% Tween-20 for 3 × 5 min, and incubated overnight at +4°C with SIRT1(no. 07-131; Millipore) primary antibodies (1:1,000). The membranes were washed with TBS-0.05% Tween-20 for 3 × 5 min before incubating them with secondary anti-rabbit horseradish peroxidase–conjugated immunoglobulin (no. NA934V; GE Health Care, Amersham, U.K.) (1:40,000) for 2 h at room temperature. The membranes were finally washed with TBS-0.05% Tween-20 for 3 × 5 min. For GAPDH, the membranes were blocked in TBS with 5% milk and 0.1% Tween-20 for 2 h at room temperature, washed with TBS-0.1% Tween-20 for 3 × 5 min, and incubated with GAPDH (no. ab8245; Abcam) primary antibodies (1:5,000) overnight at +4°C. The membranes were washed with TBS-0.1% Tween-20 for 3 × 5 min before incubating them with secondary anti-mouse horseradish peroxidase–conjugated immunoglobulin (no. NA931V; GE Health Care) (1:10,000) in TBS-0.1% Tween-20 for 1 h at room temperature. The membranes were finally washed with TBS-0.1% Tween-20 for 3 × 5 min. The bands were visualized using chemiluminescence (ECL plus; GE Health Care), and images were captured in a Image Quant RT-ECL machine (version 1.0.1; GE Health Care). Quantification of the bands was done by applying Quantity One software (Bio-Rad). Sirt1 protein expression was normalized to GAPDH protein levels. The experiments were repeated four times.
Statistical analysis.
Data analyses were carried out with SPSS 14.0 for Windows. The results for continuous variables are given as means ± SD. Variables with skewed distribution (glucose, insulin, FFAs, and subcutaneous and intra-abdominal fat) were logarithmically transformed for statistical analyses. Linear regression was used to calculate the correlations. Uni-and multivariate regression models were applied to assess the determinants of the rates of the WBGU. Linear mixed-model analysis was applied to adjust for confounding factors. For mixed-model analysis, we included the pedigree (coded as a family number) as a random factor, the tertiles as fixed factors, and age as a covariate.
RESULTS
Energy expenditure and insulin sensitivity.
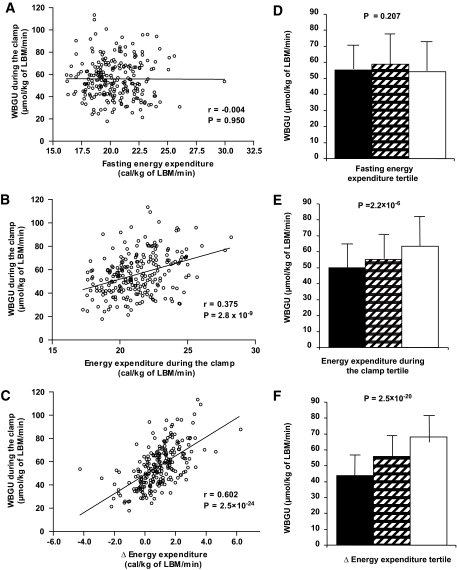
EE during the clamp positively correlated with insulin sensitivity (r = 0.375, P = 2.8 × 10−9) (Fig. 1). Even stronger correlation was found between insulin-stimulated energy expenditure ΔEE (defined as EE during the clamp − EE in the fasting state) and insulin sensitivity (r = 0.602, P = 2.5 × 10−24). In contrast, fasting EE was not correlated with insulin sensitivity (r = −0.004). To further investigate the association of EE and insulin sensitivity, we analyzed the rates of WBGU/LBM during the hyperinsulinemic clamp according to the tertiles of EE (Fig. 1). We did not find differences in WBGU among the tertiles of fasting EE, glucose oxidation, or nonoxidative glucose disposal (data not shown). In contrast, subjects in the highest tertile of EE/LBM during the hyperinsulinemic clamp had highest WBGU/LBM (49.85 ± 15.43 vs. 55.02 ± 15.46 vs. 63.44 ± 18.76 μmol/kg LBM/min, P = 2.2 × 10−6), which was attributable to both high-glucose oxidative (19.54 ± 5.42 vs. 20.96 ± 5.62 vs. 22.75 ± 6.11 μmol/kg LBM/min, P = 0.007) and nonoxidative (30.31 ± 12.81 vs. 34.06 ± 13.24 vs. 40.68 ± 16.52 μmol/kg LBM/min, P = 1.2 × 10−5) glucose disposal. These differences were even more pronounced across the tertiles of ΔEE/LBM, where subjects in the highest tertile had highest WBGU/LBM (43.82 ± 13.25 vs. 55.75 ± 13.64 vs. 67.96 ± 16.31 μmol/kg LBM/min, P = 2.5 × 10−20), attributable to both high-glucose oxidative (17.51 ± 4.34 vs. 20.81 ± 5.45 vs. 24.58 ± 5.31 μmol/kg LBM/min, P = 5.9 × 10−16) and nonoxidative (26.31 ± 12.08 vs. 34.94 ± 12.24 vs. 43.38 ± 15.16 μmol/kg LBM/min, P = 3.6 × 10−13) glucose disposal.
FIG. 1.
A: Correlation between the rates of WBGU and fasting EE (univariate linear regression). Correlation between the rates of WBGU and EE during the hyperinsulinemic clamp (B) and correlation between the rates of WBGU and ΔEE (defined as EE during the clamp − EE in the fasting state) (C). Rates of WBGU in the lowest (■), middle (▨), and highest (□) EE tertiles according to fasting EE (D), EE during the hyperinsulinemic clamp (E), and the ΔEE (F). Data are means ± SD (D–F).
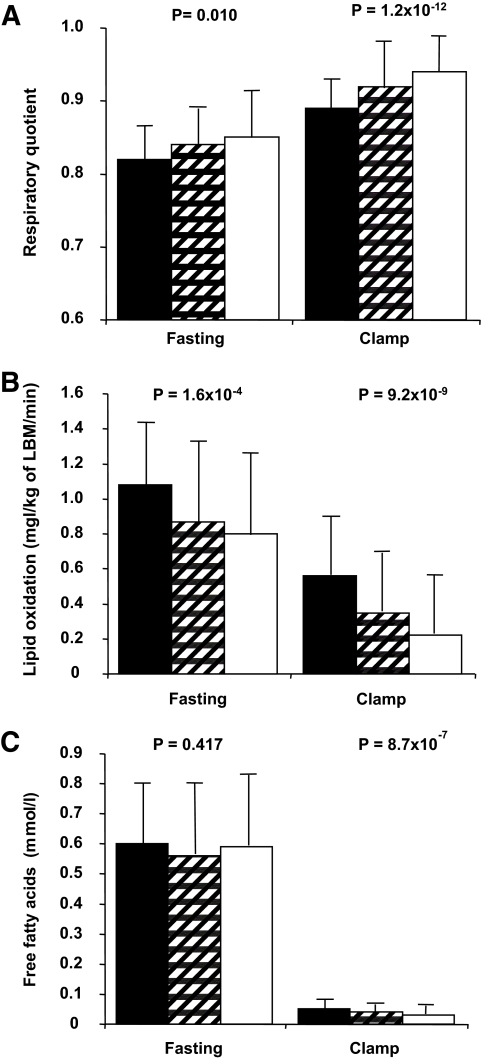
Subjects in the highest ΔEE tertile used more glucose for energy production than did subjects in the lower ΔEE tertiles, as indicated by their higher respiratory quotient in the fasting state (P = 0.010) and during the hyperinsulinemic clamp (P = 1.2 × 10−12) (Fig. 2). Subjects with the highest ΔEE had the lowest lipid oxidation in the fasting state (P = 1.6 × 10−4) and during the hyperinsulinemic clamp (P = 9.2 × 10−9). In the fasting state, FFA levels were not different among the tertiles (P = 0.417), whereas during the hyperinsulinemic clamp, subjects with the highest ΔEE had the lowest levels of FFAs (0.05 ± 0.03 vs. 0.04 ± 0.02 vs. 0.03 ± 0.03 mmol/l, P = 8.7 × 10−7).
FIG. 2.
A: Respiratory quotient in the fasting state and during the hyperinsulinemic-euglycemic clamp in the tertiles of ΔEE. Data are means ± SD in the lowest (■), middle (▨), and highest (□) tertile. Lipid oxidation in the fasting state and during the hyperinsulinemic-euglycemic clamp in the tertiles of ΔEE (B) and FFAs in the fasting state and during the hyperinsulinemic-euglycemic clamp in the tertiles of ΔEE (C). Data are means ± SD.
To evaluate variables associated with the rates of WBGU/LBM during the hyperinsulinemic clamp, we performed univariate linear regression analysis (Table 1). High ΔEE exhibited the strongest association with high WBGU/LBM, followed by low levels of low intra-abdominal adipose tissue mass and low total triglycerides. Low lipid oxidation during the hyperinsulinemic clamp and low subcutaneus adipose tissue mass were also associated with insulin sensitivity. In multivariate regression analyses, a model including ΔEE and intra-abdominal adipose tissue as independent variables explained a higher proportion of the variance of WBGU/LBM (R2 = 0.431, P = 1.7 × 10−24) than did ΔEE alone (R2 = 0.362), but adding age and sex into this model did not improve substantially the R2 value (R2 = 0.436, P = 5.4 × 10−23). A model that included ΔEE and subcutaneous adipose tissue mass as independent variables was not more strongly associated with WBGU/LBM (R2 = 0.379, P = 8.0 × 10−21) than was ΔEE alone.
TABLE 1.
Variables associated with the rates of WBGU/LBM (univariate linear regression model, n = 247)
| Independent variable | Standardized coefficient | R2 | P value |
|---|---|---|---|
| ΔEE (clamp − fasting) | 0.602 | 0.362 | 2.5 × 10−24 |
| Intra-abdominal adipose tissue | −0.497 | 0.247 | 3.1 × 10−14 |
| Total triglycerides | −0.480 | 0.230 | 1.3 × 10−15 |
| Lipid oxidation/LBM during the clamp | −0.438 | 0.192 | 1.7 × 10−12 |
| Subcutaneus adipose tissue | −0.338 | 0.114 | 6.8 × 10−7 |
| EE/LBM in the fasting state | −0.004 | 0.000 | 0.946 |
SIRT1 mRNA expression correlation with EE, insulin sensitivity, and SIRT1 target genes.
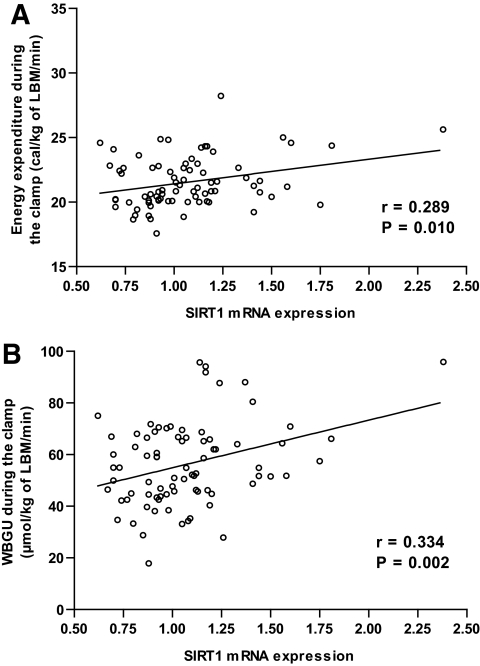
To explore the determinants of insulin-stimulated EE and WBGU/LBM, we measured adipose tissue mRNA expression of SIRT1 and PGC-1α. SIRT1 mRNA expression correlated significantly with EE (r = 0.289, P = 0.010) and with WBGU/LBM (r = 0.334, P = 0.002) during the euglycemic clamp (Fig. 3). No statistically significant correlation was found between SIRT1 expression and EE in the fasting state (r = 0.142). The correlation between SIRT1 expression and PGC-1α expression was 0.448 (P = 3.5 ×10−5). PGC-1α expression correlated significantly only with WBGU/LBM (r = 0.387, P = 3.9 ×10−4) but not with EE during the clamp (r = 0.167).
FIG. 3.
A: Correlation of adipose tissue SIRT1 mRNA expression level with EE during the hyperinsulinemic clamp. B: Correlation of adipose tissue SIRT1 mRNA expression level with the rates of whole-body glucose uptake in offspring of type 2 diabetic patients.
We also measured adipose tissue mRNA levels of several target genes of SIRT1 and PGC-1α (Table 2). SIRT1 mRNA expression correlated significantly with PGC-1β expression, estrogen-related receptor (ERR) α, nuclear respiratory factor-1 (NRF-1), mitochondrial transcription factor A (TFAM), catalase (CAT), and with several genes of the respiratory chain, including NADH dehydrogenase (ubiquinone) 1α subcomplex 2, cytochrome c, cytochrome c oxidase subunit IV, and ATP synthase. SIRT1 mRNA expression also correlated with the expression of soluble superoxide dismutase 1. The correlations of mRNA expression of these genes with PGC-1α expression were quite similar but somewhat weaker. Neither SIRT1 mRNA expression nor PGC-1α mRNA expression correlated with superoxide dismutase 2.
TABLE 2.
Pearson correlations between adipose tissue mRNA expression of SIRT1 and PGC-1α with adipose tissue mRNA expression of genes regulating mitochondrial function (n = 81)
| SIRT1 | PGC-1α | |
|---|---|---|
| PGC-1β | r = 0.358 | r = 0.152 |
| P = 0.001 | P = 0.179 | |
| NRF1 | r = 0.286 | r = 0.235 |
| P = 0.010 | P = 0.036 | |
| ERRα | r = 0.339 | r = 0.260 |
| P = 0.002 | P = 0.021 | |
| TFAM | r = 0.379 | r = 0.213 |
| P = 0.001 | P = 0.059 | |
| NDUFA2 | r = 0.392 | r = 0.273 |
| P = 3.5 × 10−4 | P = 0.015 | |
| CYCS | r = 0.263 | r = 0.159 |
| P = 0.019 | P = 0.161 | |
| COX4I1 | r = 0.332 | r = 0.262 |
| P = 0.003 | P = 0.020 | |
| ATP5G1 | r = 0.248 | r = 0.196 |
| P = 0.027 | P = 0.084 | |
| SOD1 | r = 0.460 | r = 0.348 |
| P = 2.0 × 10−5 | P = 0.002 | |
| SOD2 | r = −0.046 | r = −0.009 |
| P = 0.689 | P = 0.940 | |
| CAT | r = 0.350 | r = 0.422 |
| P = 0.002 | P = 1.3 × 10−4 |
Expression of all genes was normalized to RPL0 expression. ATP5G1, ATP synthase, H + transporting, mitochondrial F0 complex, subunit C1; CAT, catalase; COX4I1, cytochrome c oxidase subunit IV isoform 1; CYCS, cytochrome c, somatic; NDUFA2, NADH dehydrogenase (ubiquinone) 1 α subcomplex 2; SOD1, superoxide dismutase 1, soluble; SOD2, superoxide dismutase 2, mitochondrial.
SIRT1 mRNA expression correlation in adipose tissue and skeletal muscle.
In 11 subjects who had both adipose tissue and skeletal muscle biopsy, the correlation of SIRT1 mRNA expression in these tissues was 0.655 (P = 0.029). Mitochrondial DNA in skeletal muscle also correlated positively with SIRT mRNA expression in adipose tissue (r = 0.519) and skeletal muscle (r = 0.533), although the correlations were not statistically significant due to a small sample size (supplemental Table 2 in the online appendix [available at http://diabetes.diabetesjournals.org/cgi/content/full/db09-1191/DC1]).
SIRT1 mRNA expression correlation with cytokines and adhesion molecules.
SIRT mRNA expression negatively correlated with hs-CRP (r = −0.241, P = 0.039), but otherwise the correlations with cytokines and adhesion molecules were almost entirely nonsignificant (supplementary Table 1).
Sirt1 mRNA and protein correlation.
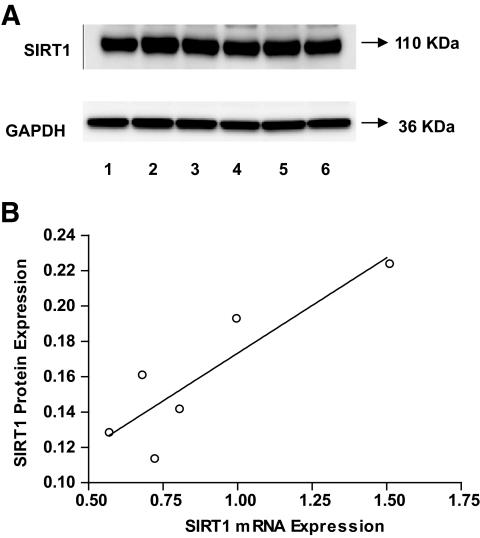
Subcutaneous adipose tissue from 5-month-old female mice was obtained and used for quantitative RT-PCR and Western blot analyses. We observed a strong correlation between Sirt1 mRNA and Sirt1 protein expression levels (r = 0.882, P < 0.001) (Fig. 4).
FIG. 4.
A: Sirt1 protein expression Western blots in subcutaneous adipose tissue from six 5-month-old female mice (numbered from 1 to 6). GAPDH was used as a loading control. B: Correlation of Sirt1 mRNA expression level with Sirt1 protein expression level (r = 0.882, P = 0.020). A mean value of triplicates was used for Sirt1 mRNA level.
DISCUSSION
Our study demonstrated that insulin-stimulated increase in EE was strongly associated with insulin sensitivity in offspring of patients with type 2 diabetes. Furthermore, we showed for the first time that adipose tissue SIRT1 mRNA expression correlated with EE and insulin sensitivity during hyperinsulinemia. Moreover, SIRT1 expression correlated with the expression of several genes regulating the mitochondrial function.
In our study, high EE during hyperinsulinemia, and particularly high ΔEE, were strongly associated with insulin-stimulated WBGU/LBM. These results agree with previous studies (16,17) including relatively small samples of lean and obese subjects. Hyperinsulinemic clamp simulates the postprandial state with high insulin levels that promote the glucose flux from circulating blood into insulin sensitive tissues. An 8-h insulin infusion in humans has been shown to increase mitochondrial mRNA transcript levels, mitochondrial protein synthesis, and ATP production (18). This response was, however, blunted in type 2 diabetic patients. In another study (19), diabetic patients exhibited lower ATP production rate in response to high-dose insulin infusion compared with that in nondiabetic individuals. Thus, impaired mitochondrial fitness could be a consequence of impaired insulin action as supported by a study in mice fed a high-fat, high-sucrose diet showing that mitochondrial alterations do not precede the onset of insulin resistance (20). In agreement with this notion, a recent study (21) in mice demonstrated a direct effect of SIRT1 on insulin sensitivity by repressing PTP1B. Whether this mechanism is working also in humans needs to be shown.
Alternatively, primary mitochondrial dysfunction could lead to insulin resistance. An attractive possibility to explain a causal link between impaired mitochondrial function and insulin resistance is the hypothesis that impaired OXPHOS capacity leads to intramyocellular lipid accumulation (9) and thus impaired insulin signaling and insulin resistance (22). High lipid levels in the circulating blood impair insulin-stimulated ATP production in humans (23). We observed that subjects with low insulin-stimulated EE also had higher levels of FFAs, higher lipid oxidation, and lower respiratory quotient during the hyperinsulinemic clamp, reflecting changes in fuel selection in these subjects, which often lead to insulin resistance.
Further evidence supporting the hypothesis that mitochondrial activity stimulated by SIRT1 might be important for energy metabolism and insulin action are high correlations of adipose tissue SIRT1 mRNA expression with expression of genes regulating mitochondrial function. SIRT1 mRNA expression correlated significantly with PGC-1β expression, which has several overlapping functions with PGC-1α in inducing genes related to OXPHOS (24). Significant correlations of SIRT1 mRNA expression were also observed with ERRα, which mediates many of the downstream effects of activated PGC-1α on mitochondrial function (25), with NRF-1, which is an ERRα/PGC-1α target, and with TFAM, which is a target of NRF-1 (26). Furthermore, SIRT1 mRNA levels correlated with expression of several genes regulating the respiratory chain. SIRT1 mRNA expression level also correlated with the expression of soluble superoxide dismutase 1, which protects the cell from superoxide toxicity, and with catalase, which catalyzes the decomposition of hydrogen peroxide to water and oxygen (27). Because the accumulation of reactive oxygen species can induce insulin resistance (28), the role of SIRT1 in governing superoxide dismutase 1 and catalase expression could cooperate with the effects of SIRT1 on the control of OXPHOS to improve insulin sensitivity. Our findings are also in agreement with recent studies in mice (4,29). Transgenic mice overexpressing SIRT1 (30) or mice having SIRT1 activity enhanced by the administration of the SIRT1 agonist, resveratrol (4,29), or a small molecule activator of SIRT1 (6), were both leaner, more hypermetabolic, and showed favorable effects on glucose and lipid metabolism. Mice treated with SRT1720, a potent synthetic activator of SIRT1, enhanced insulin sensitivity (31). Furthermore, in human studies a SIRT1 activator (SRT501) has been shown to improve glucose control (32). Adipose tissue SIRT1 mRNA expression was negatively associated with hs-CRP level, which is in agreement with the anti-inflammatory effect of SIRT1 (33). The correlation of adipose tissue SIRT1 mRNA with other inflammatory markers was modest. This may reflect a poorer correlation of SIRT1 mRNA with plasma levels of cytokines compared with cytokine expression in adipose tissue.
SIRT1 mRNA expression in adipose tissue had a high correlation with skeletal muscle SIRT1 mRNA (r = 0.655). Therefore, we believe that our results obtained in adipose tissue reflect metabolic changes in skeletal muscle, which is the main tissue for EE and insulin sensitivity during insulin stimulation. Skeletal muscle mitochondrial DNA correlated closely with SIRT1 mRNA expression in skeletal muscle and adipose tissue, giving evidence that upregulation of the genes regulating mitochondrial biogenesis in adipose tissue and likely to reflect corresponding changes in skeletal muscle (Table 2). Finally, we demonstrated in mice that Sirt mRNA expression and Sirt1 protein levels were highly correlated (Fig. 4), demonstrating that our results are likely to be valid also at protein level. However, the limitation of our study is that we could not determine SIRT1 protein level from adipose tissue biopsies due to a small amount of tissue that we can obtain using needle biopsy techniques.
In summary, we demonstrated that insulin-stimulated EE is strongly associated with insulin-stimulated glucose uptake in offspring of subjects with type 2 diabetes. Impaired stimulation of EE by insulin is likely to reflect impaired regulation of mitochondrial function in insulin-resistant states. This could be at least partially explained by low expression of SIRT1 and PGC-1α, two important master regulators of mitochondrial activity. Even though it is not possible to determine the primary defect from our cross-sectional data, disturbance in mitochondrial function and low EE were strongly associated with impaired insulin-stimulated glucose uptake. Our results give evidence that activating SIRT1 could be one of the potential mechanisms to treat insulin resistance and patients with type 2 diabetes.
Supplementary Material
ACKNOWLEDGMENTS
Work in the Laakso laboratory was supported by the Academy of Finland and the EU FP6 (EUGENE2; LSHM-CT-2004-512013). Work in the Auwerx laboratory was supported by grants from the Centre National de la Recherche Scientifique, the Institut National de la Santé et de la Recherche Médicale, the Université Louis Pasteur, the Ecole Polytechnique Fédérale de Lausanne, the National Institutes of Health (DK069966), and the EU FP6 (EUGENE2; LSHM-CT-2004-512013). H.Y. was supported by the Fondation Recherche Médicale.
D.A.S. is a consultant to Sirtris Pharmaceuticals (a GlaxoSmithKline company developing sirtuin-based drugs) and an inventor on Harvard patents licensed to GlaxoSmithKline. P.E. was and C.W. is employed by Sirtris Pharmaceuticals. No other potential conflicts of interest relevant to this article were reported.
The authors thank the members of the Laakso and Auwerx laboratories for discussions and technical assistance.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Morino K, Petersen KF, Dufour S, Befroy D, Frattini J, Shatzkes N, Neschen S, White MF, Bilz S, Sono S, Pypaert M, Shulman GI: Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest 2005; 115: 3587– 3593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kahn SE, Hull RL, Utzschneider KM: Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006; 444: 840– 846 [DOI] [PubMed] [Google Scholar]
- 3.Toledo FG, Menshikova EV, Ritov VB, Azuma K, Radikova Z, Delany J, Kelley DE: Effects of physical activity and weight loss on skeletal muscle mitochondria and relationship to glucose control in type 2 diabetes mellitus. Diabetes 2007; 56: 2142– 2147 [DOI] [PubMed] [Google Scholar]
- 4.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J: Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 2006; 127: 1109– 1122 [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto H, Schoonjans K, Auwerx J: Sirtuin functions in health and disease. Mol Endocrinol 2007; 21: 1745– 1755 [DOI] [PubMed] [Google Scholar]
- 6.Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, Bemis JE, Xie R, Disch JS, Ng PY, Nunes JJ, Lynch AV, Yang H, Galonek H, Israelian K, Choy W, Iffland A, Lavu S, Medvedik O, Sinclair DA, Olefsky JM, Jirousek MR, Elliott PJ, Westphal CH: Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature 2007; 450: 712– 716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pirinen E, Kuulasmaa T, Pietilä M, Heikkinen S, Tusa M, Itkonen P, Boman S, Skommer J, Virkamäki A, Hohtola E, Kettunen M, Fatrai S, Kansanen E, Koota S, Niiranen K, Parkkinen J, Levonen AL, Ylä-Herttuala S, Hiltunen JK, Alhonen L, Smith U, Jänne J, Laakso M: Enhanced polyamine catabolism alters homeostatic control of white adipose tissue and energy and glucose metabolism. Mol Cell Biol 2007; 27: 4953– 4967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P: Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature 2005; 434: 113– 118 [DOI] [PubMed] [Google Scholar]
- 9.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI: Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med 2004; 350: 664– 671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstrale M, Laurila E, Houstis N, Daly MJ, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D, Groop LC: PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 2003; 34: 267– 273 [DOI] [PubMed] [Google Scholar]
- 11.Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R, Landaker EJ, Goldfine AB, Mun E, DeFronzo R, Finlayson J, Kahn CR, Mandarino LJ: Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc Natl Acad Sci U S A 2003; 100: 8466– 8471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laakso M, Zilinskaite J, Hansen T, Boesgaard TW, Vänttinen M, Stancakova A, Jansson PA, Pellme F, Holst JJ, Kuulasmaa T, Hribal ML, Sesti G, Stefan N, Fritsche A, Haring H, Pedersen O, Smith U: the EUGENE2 Consortium. Insulin sensitivity, insulin release and glucagon-like peptide-1 levels in persons with impaired fasting glucose and/or impaired glucose tolerance in the EUGENE2 study. Diabetologia 2008; 51: 502– 511 [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization Definition, Diagnosis, and Classification of Diabetes Mellitus and Its Complications. Report of a WHO Consultation. Part 1: Diagnosis and Classification of Diabetes Mellitus Geneva, World Health Org., 1999. ( publ. no. WHO/NCD/NCS/99.2) [Google Scholar]
- 14.Salmenniemi U, Ruotsalainen E, Pihlajamäki J, Vauhkonen I, Kainulainen S, Punnonen K, Vanninen E, Laakso M: Multiple abnormalities in glucose and energy metabolism and coordinated changes in levels of adiponectin, cytokines, and adhesion molecules in subjects with metabolic syndrome. Circulation 2004; 110: 3842– 3848 [DOI] [PubMed] [Google Scholar]
- 15.Ferrannini E: The theoretical bases of indirect calorimetry: a review. Metabolism 1988; 37: 287– 301 [DOI] [PubMed] [Google Scholar]
- 16.Ravussin E, Bogardus C, Schwartz RS, Robbins DC, Wolfe RR, Horton ES, Danforth E, Jr, Sims EA: Thermic effect of infused glucose and insulin in man: decreased response with increased insulin resistance in obesity and noninsulin-dependent diabetes mellitus. J Clin Invest 1983; 72: 893– 902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Segal KR, Albu J, Chun A, Edano A, Legaspi B, Pi-Sunyer FX: Independent effects of obesity and insulin resistance on postprandial thermogenesis in men. J Clin Invest 1992; 89: 824– 833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stump CS, Short KR, Bigelow ML, Schimke JM, Nair KS: Effect of insulin on human skeletal muscle mitochondrial ATP production, protein synthesis, and mRNA transcripts. Proc Natl Acad Sci U S A 2003; 100: 7996– 8001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asmann YW, Stump CS, Short KR, Coenen-Schimke JM, Guo Z, Bigelow ML, Nair KS: Skeletal muscle mitochondrial functions, mitochondrial DNA copy numbers, and gene transcript profiles in type 2 diabetic and nondiabetic subjects at equal levels of low or high insulin and euglycemia. Diabetes 2006; 55: 3309– 3319 [DOI] [PubMed] [Google Scholar]
- 20.Bonnard C, Durand A, Peyrol S, Chanseaume E, Chauvin MA, Morio B, Vidal H, Riesset J: Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J Clin Invest 2008; 118: 789– 800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun C, Zhang F, Ge X, Yan T, Chen X, Shi X, Zhai Q: SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab 2007; 6: 307– 319 [DOI] [PubMed] [Google Scholar]
- 22.Petersen KF, Dufour S, Shulman GI: Decreased insulin-stimulated ATP synthesis and phosphate transport in muscle of insulin-resistant offspring of type 2 diabetic parents. PLoS Med 2005; 2: e233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brehm A, Krssak M, Schmid AI, Nowotny P, Waldhausl W, Roden M: Increased lipid availability impairs insulin-stimulated ATP synthesis in human skeletal muscle. Diabetes 2006; 55: 136– 140 [PubMed] [Google Scholar]
- 24.Lin J, Puigserver P, Donovan J, Tarr P, Spiegelman BM: Peroxisome proliferator-activated receptor gamma coactivator 1beta (PGC-1beta), a novel PGC-1-related transcription coactivator associated with host cell factor. J Biol Chem 2002; 277: 1645– 1648 [DOI] [PubMed] [Google Scholar]
- 25.Tcherepanova I, Puigserver P, Norris JD, Spiegelman BM, McDonnell DP: Modulation of estrogen receptor-alpha transcriptional activity by the coactivator PGC-1. J Biol Chem 2000; 275: 16302– 16308 [DOI] [PubMed] [Google Scholar]
- 26.Larsson NG, Wang J, Wilhelmsson H, Oldfors A, Rustin P, Lewandoski M, Barsh GS, Clayton DA: Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nat Genet 1998; 8: 231– 236 [DOI] [PubMed] [Google Scholar]
- 27.St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jager S, Handschin C, Zheng K, Lin J, Yang W, Simon DK, Bachoo R, Spiegelman BM: Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 2006; 127: 397– 408 [DOI] [PubMed] [Google Scholar]
- 28.Houstis N, Rosen ED, Lander ES: Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 2006; 440: 944– 948 [DOI] [PubMed] [Google Scholar]
- 29.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA: Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006; 444: 337– 342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bordone L, Cohen D, Robinson A, Motta MC, van Veen E, Czopik A, Steele AD, Crowe H, Marmor S, Luo J, Gu W, Guarente L: SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell 2007; 6: 759– 767 [DOI] [PubMed] [Google Scholar]
- 31.Feige JN, Lagouge M, Canto C, Strehle A, Houten SM, Milne JC, Lambert PD, Mataki C, Elliott PJ, Auwerx J: Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab 2008; 8: 347– 358 [DOI] [PubMed] [Google Scholar]
- 32.Elliott PJ, Jirousek M: Sirtuins: novel targets for metabolic disease. Curr Opin Investig Drugs 2008; 9: 371– 378 [PubMed] [Google Scholar]
- 33.Yeung F, Hoberg JE, Ramsay CS, Keller MD, Jones DR, Frye RA, Mayo MW: Modulation of NFκ-B-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J 2004; 23: 2369– 2380 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.