Figure 4.
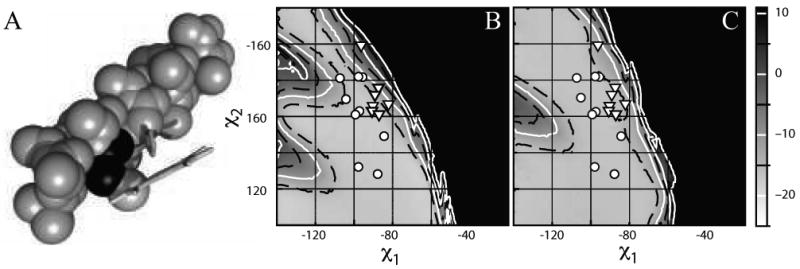
Heme ruffling is a consequence of steric clash between the heme C(γ) and the helix i-4 side chain. (A) Space-filling model of a 17-residue idealized alanine helix with a central, flat porphyrin-bound histidine fixed at the mean χ1 and χ2 torsion angles for the m166 rotamer. The alanine 4 residues N-terminal from the histidine, which clashes with the porphyrin C(γ) atom, is shaded in dark grey. (B) Expansion of the Van der Walls energy surface near the m166 rotamer. The χ1 and χ2 angles of each heme-bound histidine in the database are overlaid in white as a function of the connectivity of the heme: ((x025CB)) b-type, noncovalently bound hemes, ((x025BD)) c-type, covalently bound hemes. (C) Van der Waals energy surface calculated using the ruffled porphyrin derived from the 0.91 Ǻ resolution structure of the D. vulgaris cytochrome C (1J0P) connected as above to the idealized 17-residue alanine helix used in part B. Histidine rotamers from the database are overlaid in white as in part B.
