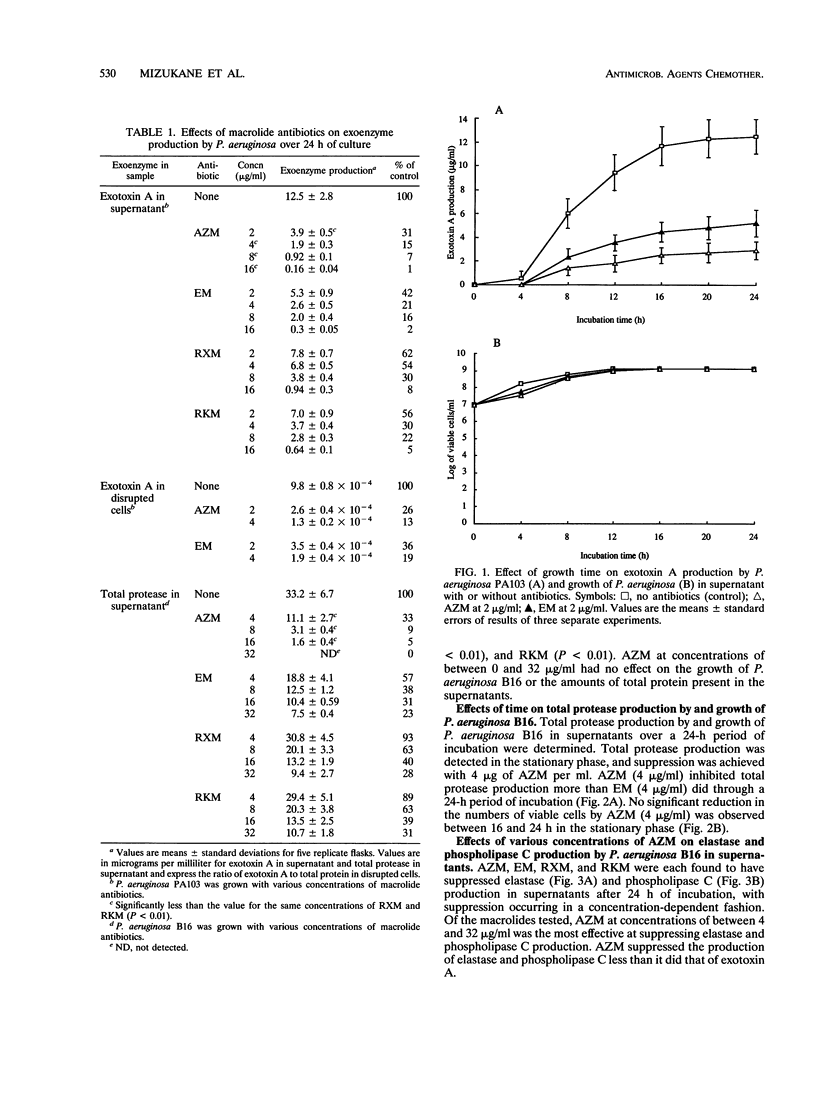
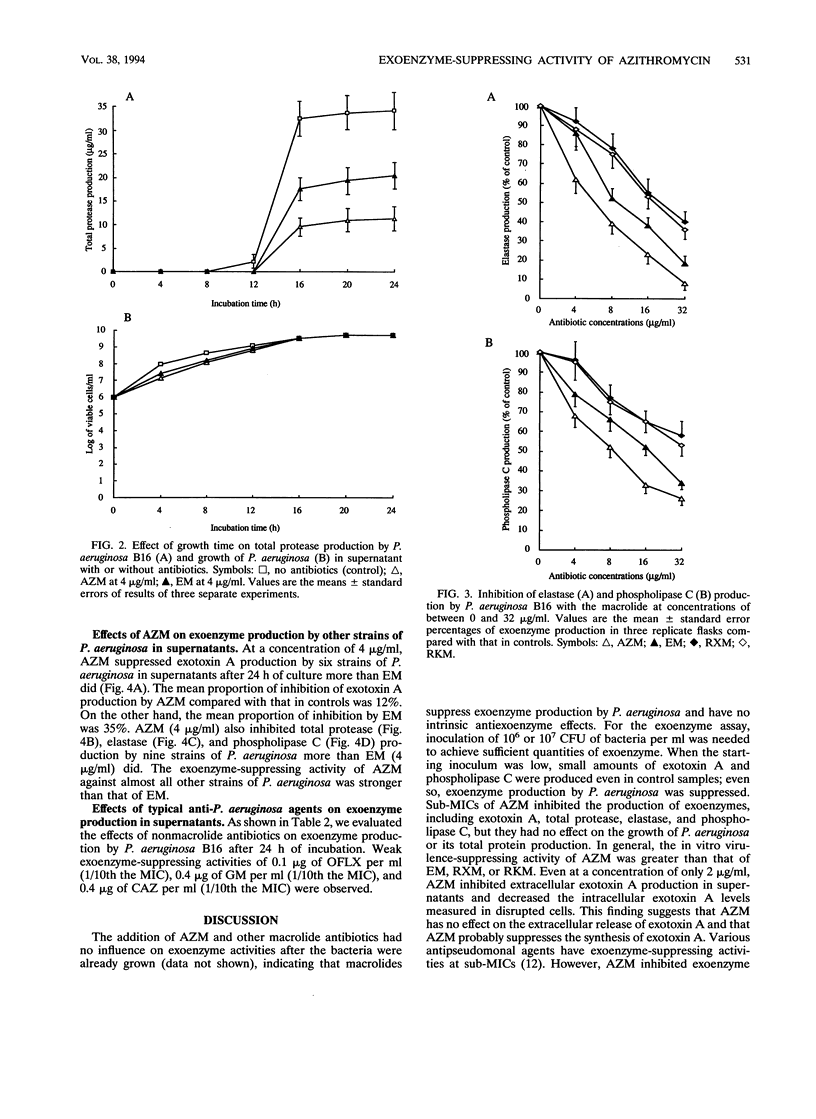
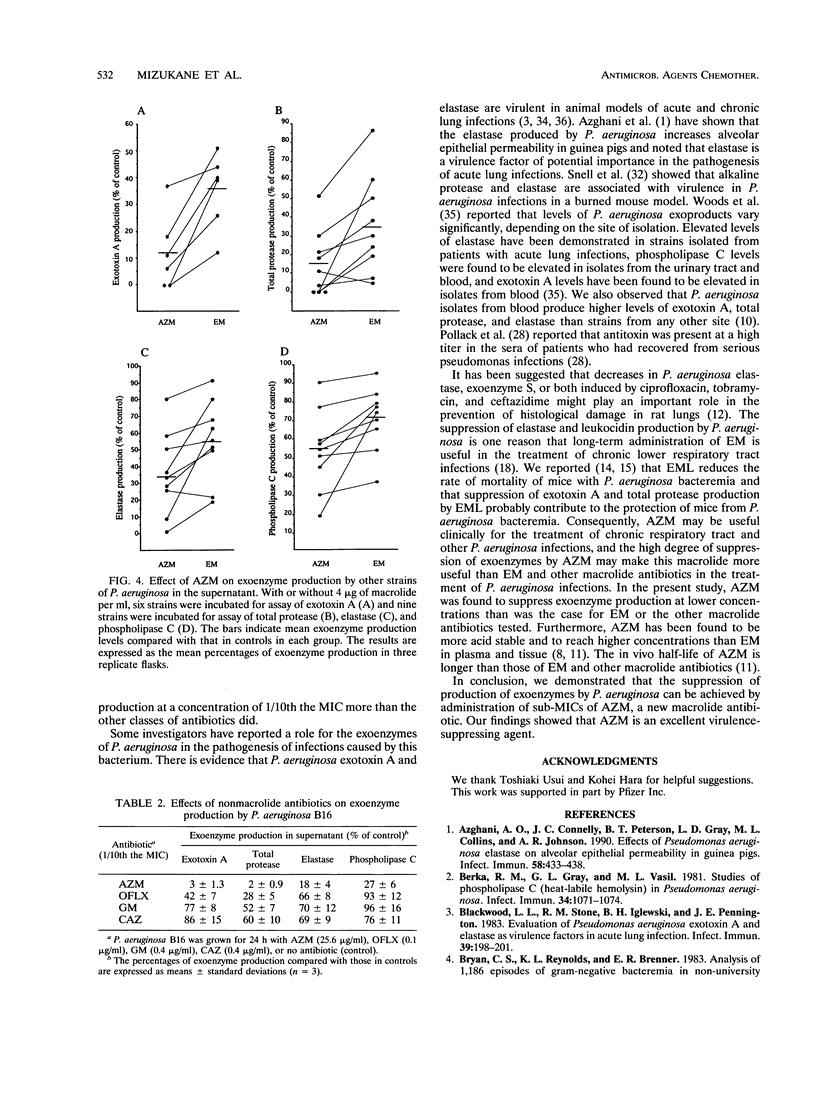
Abstract
The inhibitory effects of azithromycin (AZM), a new 15-membered macrolide antibiotic, on the production of exotoxin A, total protease, elastase, and phospholipase C by Pseudomonas aeruginosa were determined, and the virulence-suppressing effects of AZM were compared with those of erythromycin (EM), roxithromycin (RXM), and rokitamycin (RKM). The effect of exposure of P. aeruginosa PA103 or B16 in cultures to sub-MICs of these macrolide antibiotics on the production of exoenzymes was determined. AZM suppressed the in vitro production of extracellular and intracellular exotoxin A by P. aeruginosa PA103 more than did EM, even at a concentration of only 2 micrograms/ml. At concentrations of between 4 and 32 micrograms/ml, AZM also inhibited total protease, elastase, and phospholipase C production by P. aeruginosa B16 more than did EM, RXM, and RKM. AZM was effective in suppressing exotoxin A and total protease production through 24 h of incubation in the presence of drug at sub-MICs, but it had no significant effect on either the growth of P. aeruginosa or its total protein production. Moreover, at a concentration of 4 micrograms/ml, AZM suppressed exoenzyme production by other strains of P. aeruginosa more than did EM. These findings indicate that AZM, EM, RXM, and RKM each has an inhibitory effect on exoenzyme production separate from the antimicrobial effect and that, of these macrolides, AZM has the strongest virulence-suppressing effect.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azghani A. O., Connelly J. C., Peterson B. T., Gray L. D., Collins M. L., Johnson A. R. Effects of Pseudomonas aeruginosa elastase on alveolar epithelial permeability in guinea pigs. Infect Immun. 1990 Feb;58(2):433–438. doi: 10.1128/iai.58.2.433-438.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berka R. M., Gray G. L., Vasil M. L. Studies of phospholipase C (heat-labile hemolysin) in Pseudomonas aeruginosa. Infect Immun. 1981 Dec;34(3):1071–1074. doi: 10.1128/iai.34.3.1071-1074.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwood L. L., Stone R. M., Iglewski B. H., Pennington J. E. Evaluation of Pseudomonas aeruginosa exotoxin A and elastase as virulence factors in acute lung infection. Infect Immun. 1983 Jan;39(1):198–201. doi: 10.1128/iai.39.1.198-201.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan C. S., Reynolds K. L., Brenner E. R. Analysis of 1,186 episodes of gram-negative bacteremia in non-university hospitals: the effects of antimicrobial therapy. Rev Infect Dis. 1983 Jul-Aug;5(4):629–638. doi: 10.1093/clinids/5.4.629. [DOI] [PubMed] [Google Scholar]
- Dick J. D., Shull V., Karp J. E., Valentine J. Bacterial and host factors affecting Pseudomonas aeruginosa colonization versus bacteremia in granulocytopenic patients. Eur J Cancer Clin Oncol. 1988;24 (Suppl 1):S47–S54. [PubMed] [Google Scholar]
- Edelstein P. H., Edelstein M. A. In vitro activity of azithromycin against clinical isolates of Legionella species. Antimicrob Agents Chemother. 1991 Jan;35(1):180–181. doi: 10.1128/aac.35.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes A. C., Anderson R., Theron A. J., Jooné G., Van Rensburg C. E. Enhancement of human polymorphonuclear leucocyte motility by erythromycin in vitro and in vivo. S Afr Med J. 1984 Aug 4;66(5):173–177. [PubMed] [Google Scholar]
- Foulds G., Shepard R. M., Johnson R. B. The pharmacokinetics of azithromycin in human serum and tissues. J Antimicrob Chemother. 1990 Jan;25 (Suppl A):73–82. doi: 10.1093/jac/25.suppl_a.73. [DOI] [PubMed] [Google Scholar]
- Fraschini F., Scaglione F., Ferrara F., Marelli O., Braga P. C., Teodori F. Evaluation of the immunostimulating activity of erythromycin in man. Chemotherapy. 1986;32(3):286–290. doi: 10.1159/000238425. [DOI] [PubMed] [Google Scholar]
- Furuya N., Hirakata Y., Tomono K., Matsumoto T., Tateda K., Kaku M., Yamaguchi K. Mortality rates amongst mice with endogenous septicaemia caused by Pseudomonas aeruginosa isolates from various clinical sources. J Med Microbiol. 1993 Aug;39(2):141–146. doi: 10.1099/00222615-39-2-141. [DOI] [PubMed] [Google Scholar]
- Girard A. E., Girard D., English A. R., Gootz T. D., Cimochowski C. R., Faiella J. A., Haskell S. L., Retsema J. A. Pharmacokinetic and in vivo studies with azithromycin (CP-62,993), a new macrolide with an extended half-life and excellent tissue distribution. Antimicrob Agents Chemother. 1987 Dec;31(12):1948–1954. doi: 10.1128/aac.31.12.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimwood K., To M., Rabin H. R., Woods D. E. Inhibition of Pseudomonas aeruginosa exoenzyme expression by subinhibitory antibiotic concentrations. Antimicrob Agents Chemother. 1989 Jan;33(1):41–47. doi: 10.1128/aac.33.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakata Y., Furuya N., Tateda K., Kaku M., Yamaguchi K. In vivo production of exotoxin A and its role in endogenous Pseudomonas aeruginosa septicemia in mice. Infect Immun. 1993 Jun;61(6):2468–2473. doi: 10.1128/iai.61.6.2468-2473.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakata Y., Kaku M., Mizukane R., Ishida K., Furuya N., Matsumoto T., Tateda K., Yamaguchi K. Potential effects of erythromycin on host defense systems and virulence of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1992 Sep;36(9):1922–1927. doi: 10.1128/aac.36.9.1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakata Y., Kaku M., Tomono K., Tateda K., Furuya N., Matsumoto T., Araki R., Yamaguchi K. Efficacy of erythromycin lactobionate for treating Pseudomonas aeruginosa bacteremia in mice. Antimicrob Agents Chemother. 1992 Jun;36(6):1198–1203. doi: 10.1128/aac.36.6.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma H., Yamanaka A., Tanimoto S., Tamura M., Chijimatsu Y., Kira S., Izumi T. Diffuse panbronchiolitis. A disease of the transitional zone of the lung. Chest. 1983 Jan;83(1):63–69. doi: 10.1378/chest.83.1.63. [DOI] [PubMed] [Google Scholar]
- Kita E., Sawaki M., Nishikawa F., Mikasa K., Yagyu Y., Takeuchi S., Yasui K., Narita N., Kashiba S. Enhanced interleukin production after long-term administration of erythromycin stearate. Pharmacology. 1990;41(4):177–183. doi: 10.1159/000138716. [DOI] [PubMed] [Google Scholar]
- Kita E., Sawaki M., Oku D., Hamuro A., Mikasa K., Konishi M., Emoto M., Takeuchi S., Narita N., Kashiba S. Suppression of virulence factors of Pseudomonas aeruginosa by erythromycin. J Antimicrob Chemother. 1991 Mar;27(3):273–284. doi: 10.1093/jac/27.3.273. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liu P. V. Exotoxins of Pseudomonas aeruginosa. I. Factors that influence the production of exotoxin A. J Infect Dis. 1973 Oct;128(4):506–513. doi: 10.1093/infdis/128.4.506. [DOI] [PubMed] [Google Scholar]
- Liu P. V. The roles of various fractions of Pseudomonas aeruginosa in its pathogenesis. 3. Identity of the lethal toxins produced in vitro and in vivo. J Infect Dis. 1966 Oct;116(4):481–489. doi: 10.1093/infdis/116.4.481. [DOI] [PubMed] [Google Scholar]
- Maskell J. P., Sefton A. M., Williams J. D. Comparative in-vitro activity of azithromycin and erythromycin against Gram-positive cocci, Haemophilus influenzae and anaerobes. J Antimicrob Chemother. 1990 Jan;25 (Suppl A):19–24. doi: 10.1093/jac/25.suppl_a.19. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Chin N. X., Saha G., Labthavikul P. Comparative in vitro activity of the new oral macrolide azithromycin. Eur J Clin Microbiol Infect Dis. 1988 Aug;7(4):541–544. doi: 10.1007/BF01962611. [DOI] [PubMed] [Google Scholar]
- Neu H. C. The role of Pseudomonas aeruginosa in infections. J Antimicrob Chemother. 1983 May;11 (Suppl B):1–13. doi: 10.1093/jac/11.suppl_b.1. [DOI] [PubMed] [Google Scholar]
- Nicas T. I., Iglewski B. H. The contribution of exoproducts to virulence of Pseudomonas aeruginosa. Can J Microbiol. 1985 Apr;31(4):387–392. doi: 10.1139/m85-074. [DOI] [PubMed] [Google Scholar]
- Ohman D. E., Sadoff J. C., Iglewski B. H. Toxin A-deficient mutants of Pseudomonas aeruginosa PA103: isolation and characterization. Infect Immun. 1980 Jun;28(3):899–908. doi: 10.1128/iai.28.3.899-908.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack M., Callahan L. T., 3rd, Taylor N. S. Neutralizing antibody to Pseudomonas aeruginosa exotoxin in human sera: evidence for in vivo toxin production during infection. Infect Immun. 1976 Oct;14(4):942–947. doi: 10.1128/iai.14.4.942-947.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retsema J., Girard A., Schelkly W., Manousos M., Anderson M., Bright G., Borovoy R., Brennan L., Mason R. Spectrum and mode of action of azithromycin (CP-62,993), a new 15-membered-ring macrolide with improved potency against gram-negative organisms. Antimicrob Agents Chemother. 1987 Dec;31(12):1939–1947. doi: 10.1128/aac.31.12.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rylander M., Hallander H. O. In vitro comparison of the activity of doxycycline, tetracycline, erythromycin and a new macrolide, CP 62993, against Mycoplasma pneumoniae, Mycoplasma hominis and Ureaplasma urealyticum. Scand J Infect Dis Suppl. 1988;53:12–17. [PubMed] [Google Scholar]
- SACHAR L. A., WINTER K. K., SICHER N., FRANKEL S. Photometric method for estimation of elastase activity. Proc Soc Exp Biol Med. 1955 Nov;90(2):323–326. doi: 10.3181/00379727-90-22022. [DOI] [PubMed] [Google Scholar]
- Snell K., Holder I. A., Leppla S. A., Saelinger C. B. Role of exotoxin and protease as possible virulence factors in experimental infections with Pseudomonas aeruginosa. Infect Immun. 1978 Mar;19(3):839–845. doi: 10.1128/iai.19.3.839-845.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood R. E., Boat T. F., Doershuk C. F. Cystic fibrosis. Am Rev Respir Dis. 1976 Jun;113(6):833–878. doi: 10.1164/arrd.1976.113.6.833. [DOI] [PubMed] [Google Scholar]
- Woods D. E., Cryz S. J., Friedman R. L., Iglewski B. H. Contribution of toxin A and elastase to virulence of Pseudomonas aeruginosa in chronic lung infections of rats. Infect Immun. 1982 Jun;36(3):1223–1228. doi: 10.1128/iai.36.3.1223-1228.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D. E., Schaffer M. S., Rabin H. R., Campbell G. D., Sokol P. A. Phenotypic comparison of Pseudomonas aeruginosa strains isolated from a variety of clinical sites. J Clin Microbiol. 1986 Aug;24(2):260–264. doi: 10.1128/jcm.24.2.260-264.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D. E., Sokol P. A. Role of Pseudomonas aeruginosa extracellular enzymes in lung disease. Clin Invest Med. 1986;9(2):108–112. [PubMed] [Google Scholar]


