Fig. 4.
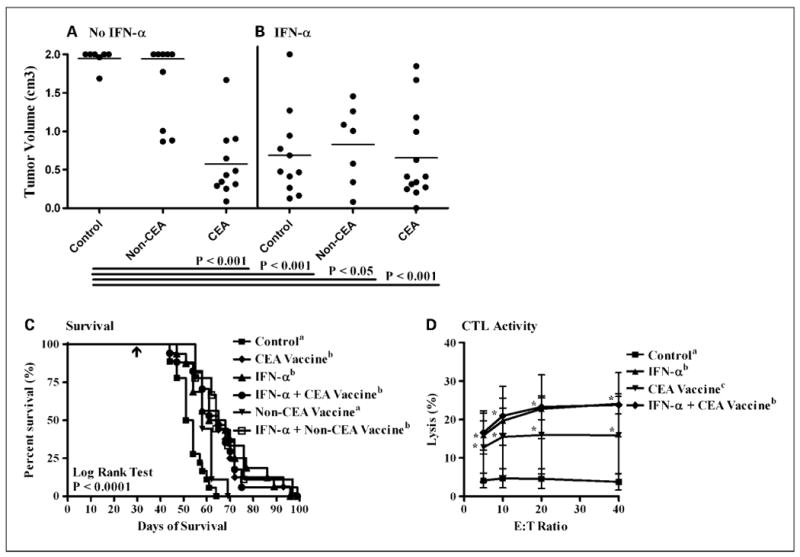
The antitumor efficacy of IFN-α combined with rV/F-CEA-TRICOM vaccination against subcutaneous murine pancreatic adenocarcinomas. Mice with established Panc02.CEA tumors were randomized into one of six treatment groups (n = 10 mice per group): untreated controls (■); rV/F-CEA-TRICOM + rF-GM-CSF (◆, CEA vaccine); IFN-α (▲, 50,000 IU/mouse, 3× a week, alternating weeks); IFN-α and rV/F-CEA-TRICOM + rF-GM-CSF (●, IFN-α + CEA vaccine); rV/F-TRICOM + rF-GM-CSF (▼, non-CEA vaccine); and IFN-α and rV/F-TRICOM + rF-GM-CSF (□, IFN-α + non-CEA vaccine). In mice with Panc02.CEA tumors, significant antitumor efficacy and improved survival were observed with rV/F-CEA-TRICOM vaccination (A and C), IFN-α monotherapy (B and C), and the combination of IFN-α and rV/F-CEA-TRICOM vaccination (B and C). C, arrow, initiation of treatment. Significant CEA-specific CTL lysis of Panc02.CEA targets was observed in mice receiving IFN-α, rV/F-CEA-TRICOM, and the combination of IFN-α and rV/F-CEA-TRICOM (D). Statistically significant differences in the mean tumor volume and percent lysis of each treatment group were compared using ANOVA and Tukey's multiple comparison test. Survival estimates were generated using Kaplan-Meier plots and the log-rank test. Statistical significance was accepted at P < 0.05 and is indicated by superscript letters.
