Abstract
OBJECTIVE
To determine the longitudinal association of components of health-related functioning (HRF) with incident impaired glucose metabolism and type 2 diabetes.
RESEARCH DESIGN AND METHODS
The Australian Diabetes Obesity and Lifestyle (AusDiab) study is a national, longitudinal study of adults aged ≥25 years from 42 randomly selected areas of Australia. Diabetes status was defined using the World Health Organization criteria, and HRF was assessed using the SF-36 questionnaire in 1999–2000 and 2004–2005.
RESULTS
Incident impaired fasting glucose (IFG), impaired glucose tolerance (IGT), and newly diagnosed type 2 diabetes were associated with increased bodily pain at baseline compared with those with normal glucose tolerance (NGT) (IFG P = 0.005, IGT P < 0.004, and newly diagnosed type 2 diabetes P = 0.005), after adjustment. In addition, those with incident IGT and newly diagnosed type 2 diabetes had significantly reduced physical functioning, general health, mental health, and vitality at baseline compared with those with NGT. After we controlled for factors associated with incident diabetes, those in the lowest quartile of the physical component summary scale at baseline had at least a 50% higher risk of progression to impaired glucose metabolism and diabetes 5 years later.
CONCLUSIONS
These findings show that incident IFG, IGT, and newly diagnosed type 2 diabetes are associated with reduced HRF independent of cardiovascular disease and that this is evident before the onset of these conditions. If future health promotion campaigns are to effectively target those at high risk of developing diabetes, an understanding of the process of declining health before onset of the disease is essential.
The prevalence of type 2 diabetes is increasing rapidly throughout the world. The difficulties in curbing this trend make it vital that we fully understand all aspects of the disease process. Although traditional risk factors for type 2 diabetes have been extensively studied, the role of components of health-related functioning (HRF), an important component of health (1), has not fully been explored in contemporary, population-based studies.
There is now growing evidence to suggest that HRF, which reflects a measure of physical, social, and mental health functioning, may be impaired before the onset of type 2 diabetes (2). A small number of cross-sectional studies have suggested that those with impaired fasting glucose (IFG) and or impaired glucose tolerance (IGT) have reduced HRF compared with those with normal glucose tolerance (NGT) on a number of the SF-36 dimensions (3–5). Although many studies have previously shown that individuals with diabetes-related complications have reduced HRF compared with those without complications (6,7) and several studies have shown that HRF is impaired among those with diabetes compared with those without diabetes (8,9), little is known about the stage of the disease at which HRF becomes impaired. This is important to establish if a holistic approach to health care is to be taken, and we are to fully understand the etiology and pathogenesis of the disease.
An understanding of how some psychosocial factors directly impact the development of type 2 diabetes and other chronic diseases is already beginning to emerge. Psychosocial stress, for example, is known to increase the inflammatory process (10,11) and has been linked directly with accelerated atherosclerosis (10), myocardial ischemia (12), and incident type 2 diabetes (2). This finding suggests that psychosocial risk factors are likely to be important to consider in the identification of individuals at high risk of developing type 2 diabetes, as well as in interventions to prevent diabetes. The Australian Diabetes, Obesity and Lifestyle (AusDiab) study, with its large, national, population-based sample, with 5 years of follow-up, provides an ideal setting in which to investigate the association of HRF with the subsequent development of impaired glucose metabolism and type 2 diabetes.
RESEARCH DESIGN AND METHODS
The population, methods and response rates of the AusDiab study are detailed elsewhere (13,14). In brief, the AusDiab study was a population-based study of 11,247 individuals aged ≥25 years, from 42 randomly selected urban and rural areas of Australia, conducted in 1999–2000. At baseline, 55.3% (n = 11,247) of those completing a household questionnaire undertook the full survey, and the follow-up response rate in 2004–2005 was 60.6% (6,537 OF 10,788). There were 459 who were ineligible for the follow-up study because of a terminal illness, death or refusal of further contact. The study was approved by the ethics committee of the International Diabetes Institute. Informed consent for the study was obtained from all participants.
Diabetes classification was based on plasma glucose results, using the 1999 World Health Organization diabetes classification (15). Diabetes was diagnosed on the basis of fasting plasma glucose of ≥7.0 mmol/l or 2-h plasma glucose of ≥11.1 mmol/l or current treatment with insulin or oral hypoglycemic medication. Those with self-reported diabetes, those receiving current treatment (insulin or oral hypoglycemic medication), and those with diabetic glucose values at baseline were categorized as having known diabetes and were excluded from this study. Newly diagnosed diabetes was defined as participants in whom diabetes was diagnosed through the AusDiab study. Incident newly diagnosed diabetes (incident diabetes) at 5 years was defined as participants without known diabetes or newly diagnosed diabetes at baseline in whom diabetes was diagnosed by the follow-up examination (n = 224). Incident IGT and IFG were defined at 5 years as participants with NGT at baseline in whom IGT or IFG was diagnosed at the 5-year follow-up examination (n = 432). Only those with NGT at baseline and follow-up (n = 4,225) were included in the comparison group for the analyses focused on categories of glucose metabolism. For these analyses, complete data were available on key variables of interest for 200 with incident newly diagnosed diabetes, 257 with incident IGT, 137 with incident IFG, and 3,906 who maintained NGT throughout. For the analysis focused on those with and without incident newly diagnosed diabetes, those with IFG and IGT at baseline were also included.
In 1999–2000, fasting plasma glucose (FPG) and 2-h plasma glucose levels were determined by the glucose oxidase method using an Olympus AU600 automated analyzer (Olympus Optical, Tokyo, Japan), and in 2004–2005, a spectrophotometric hexokinase method using a Roche Modular analyzer (Roche Diagnostics, Indianapolis, IN) was used. Remeasurement of stored baseline samples with the assay used at follow-up showed good agreement between the two assays (14). Serum triglycerides, total cholesterol, and HDL cholesterol were measured by enzymatic methods at baseline. High-performance liquid chromatography (Bio-Rad Variant Hemoglobin Testing System; Bio-Rad Laboratories, Hercules, CA) was used for glycated hemoglobin analysis with standardized conversion to A1C values (normal range 4.2–6.3%). C-peptide was measured by radioimmunoassay with human C-peptide kits (Linco, St. Charles, MO). Blood pressure was measured using a Dinamap monitor or a standard mercury sphygmomanometer. To account for any effect due to differential measurement error, manual blood pressure measurements were adjusted as described previously (16). Hypertension was defined as present if systolic blood pressure was ≥140 mmHg or diastolic blood pressure was ≥90 mmHg or the participant reported current treatment for hypertension. Height and weight were measured with the subject in light clothing by a trained observer. BMI was calculated as weight in kilograms divided by the square of height in meters. Information on smoking, medications, and history of diabetes were obtained by interview.
Components of social functioning were assessed using version 1 of the SF-36 Quality of Life Scale (17). The SF-36 (http://www.sf-36.org/tools/sf36.shtml) is a self-administered measure of perceived health status over the past week, comprising eight domains: physical functioning, role-physical, bodily pain, general health, vitality, social functioning, role-emotional, and mental health. Items for each dimension are coded, summed, and translated from worst health (0) to best health (100). Physical functioning refers to the ability to perform activities (walking, climbing stairs, bending and stretching, and lifting and carrying objects) without limitation. Role limitation (physical) refers to the limitations that reduced physical health has on the range and extent of physical activities one is able to perform. Bodily pain refers to the severity of pain and its impact on daily activities. General health is a rating of one's own health, a comparison with others' health, and proneness to illness. Vitality refers to how energetic or tired an individual feels. Social functioning refers to the impact of physical and emotional health on the ability to perform normal social activities. Role limitation (emotional) refers to limitations that emotional problems put on the range and extent of activities one could perform. Mental health refers to the degree of nervousness or calmness and happiness or sadness.
In addition, the survey established scores for two summary measures: Physical Component Summary (PCS) and Mental Health Component Summary (MCS) (17). The PCS is a summary of physical functioning, role-physical, bodily pain, and general health. The MCS is a summary measure of vitality, social functioning, role-emotional, and mental health. Both PCS and MCS are components of health that have been shown to be useful and valid measures of mental and physical health functioning relative to health profile. Overall, the SF-36 has been widely used in studies of chronic disease.
Statistical methods
Statistical analysis was performed using SPSS (version 14.0 for Windows, 2005; SPSS, Chicago, IL). Descriptive information for each of the variables was derived and distribution was assessed. Univariate associations between each dimension of the SF-36 scale and other variables of interest were assessed using ANOVA for metric variables and a χ2 test for categorical variables. Two multivariate models (ANCOVA), applied separately to each SF-36 scale allow tiered analysis of the effect of diabetes status on quality-of-life measures. Model 1 was adjusted for age and sex only. Model 2 added three further important factors to model 1: systolic blood pressure, BMI, and total cholesterol. Model 3 included SF-36 dimensions adjusted for model 2 and smoking, known cardiovascular disease (CVD), and medication for hypertension or a lipid abnormality. Marginal means are presented for each model, representing the mean values for each SF-36 scale after adjustment for covariates. P < 0.05 was considered statistically significant. The risk of being in the lowest quartile (i.e., poor quality of life) of each summary measure (physical and mental component of health) was assessed using logistic regression.
RESULTS
The characteristics of the population according to glucose tolerance status at outcome are shown in Table 1. Across categories of glucose metabolism, there were significant differences in age, systolic and diastolic blood pressure, cholesterol, triglycerides, glycemic measures, waist circumference, and BMI.
Table 1.
Baseline population characteristics of the AusDiab study participants according to diabetes status at the 5-year follow-up
| Analysis of incident IFG/IGT |
Analysis of incident diabetes |
||||||
|---|---|---|---|---|---|---|---|
| NGT 1999 and 2005 | Incident IFG | Incident IGT | P | Nondiabetic 1999 and 2005* | Incident diabetes | P | |
| n | 3,906 | 137 | 257 | 5,171 | 200 | ||
| Age (years) | 49 ± 12 | 51 ± 10 | 55 ± 13 | <0.001 | 51 ± 12 | 55 ± 12 | <0.001 |
| Sex (male) | 43 | 58 | 44 | 0.003 | 45 | 52 | 0.050 |
| FPG (mmol/l) | 5.2 ± 0.4 | 5.6 ± 0.3 | 5.4 ± 0.4 | <0.001 | 5.4 ± 0.5 | 5.9 ± 0.6 | <0.001 |
| 2-h plasma glucose (mmol/l) | 5.4 ± 1.1 | 5.7 ± 1.2 | 6.2 ± 1.0 | <0.001 | 5.8 ± 1.5 | 5.9 ± 1.8 | <0.001 |
| BMI (kg/m2) | 25.9 ± 4.3 | 28.8 ± 4.6 | 28.1 ± 5.0 | <0.001 | 26.5 ± 4.6 | 29.6 ± 5.6 | <0.001 |
| Waist circumference (cm) | 87.4 ± 12.7 | 97.5 ± 11.8 | 94.3 ± 12.9 | <0.001 | 89.4 ± 13.2 | 98.9 ± 14.6 | <0.001 |
| Lipid treatment (%) | 6 | 7 | 8 | <0.001 | 7 | 16 | <0.001 |
| HDL cholesterol (mmol/l) | 1.5 ± 0.4 | 1.3 ± 0.3 | 1.4 ± 0.4 | <0.001 | 1.5 ± 0.4 | 1.3 ± 0.4 | <0.001 |
| Total cholesterol (mmol/l) | 5.6 ± 1.0 | 5.8 ± 0.9 | 5.9 ± 1.1 | 0.001 | 5.6 ± 1.0 | 5.9 ± 1.0 | <0.001 |
| Triglycerides (mmol/l) | 1.1 (0.8–1.6) | 1.4 (1.0–2.2) | 1.5 (1.1–2.2) | <0.001 | 1.2 (0.8–1.7) | 1.7 (1.2–2.5) | <0.001 |
| Current smoker (%) | 12 | 10 | 11 | 0.730 | 11 | 15 | 0.075 |
| Hypertension (%) | 22 | 31 | 42 | <0.001 | 27 | 54 | <0.001 |
| Systolic blood pressure (mmHg) | 125 ± 16 | 131 ± 17 | 133 ± 19 | <0.001 | 127 ± 17 | 137 ± 18 | <0.001 |
| Diastolic blood pressure (mmHg) | 69 ± 11 | 73 ± 11 | 71 ± 12 | <0.001 | 70 ± 11 | 75 ± 12 | <0.001 |
Data are mean ± SD unless otherwise indicated. Those with NGT had normal glucose tolerance at baseline and follow-up. Those included with incident IFG or IGT had NGT at baseline. Those with incident diabetes were free of diabetes at baseline and had a diagnosis of newly diagnosed diabetes at follow-up. Only those with complete data on variables modeled are included.
*Includes those with IFG or IGT at baseline or follow-up.
Incident diabetes at follow-up was associated with significantly lower mean baseline scores on each of the eight SF-36 dimensions compared with those who remained in the NGT category, adjusted for age and sex (physical functioning, role-physical, bodily pain, general health, vitality, social functioning, role-emotional, and mental health) (Table 2). These differences were only partially attenuated by adjustment for BMI, systolic blood pressure, and total cholesterol at baseline. Further adjustment for known CVD, smoking, lipid abnormality, and hypertension reduced the mean values of each dimension but did not greatly alter the association.
Table 2.
Adjusted mean values of baseline SF-36 dimensions according to glucose tolerance status at 5 years: the AusDiab study
| SF-36 dimension and diabetes status | Model 1* | P | Model 2† | P | Model 3‡ | P |
|---|---|---|---|---|---|---|
| Physical functioning | ||||||
| NGT 1999 and 2005 | 86.5 ± 0.3 | Ref | 86.2 ± 0.3 | Ref | 79.7 ± 0.7 | Ref |
| Incident IFG | 83.0 ± 1.4 | 0.010 | 85.1 ± 1.3 | 0.424 | 78.5 ± 1.5 | 0.349 |
| Incident IGT | 82.7 ± 1.0 | <0.001 | 84.2 ± 1.1 | 0.048 | 78.1 ± 1.2 | 0.087 |
| Incident newly diagnosed diabetes | 78.2 ± 1.1 | <0.001 | 81.1 ± 1.1 | <0.001 | 75.6 ± 1.2 | <0.001 |
| Role limitation physical | ||||||
| NGT 1999 and 2005 | 84.8 ± 0.5 | Ref | 84.6 ± 0.5 | Ref | 76.8 ± 1.4 | Ref |
| Incident IFG | 78.5 ± 2.6 | 0.017 | 79.7 ± 2.6 | 0.066 | 71.8 ± 2.9 | 0.057 |
| Incident IGT | 80.5 ± 1.9 | 0.031 | 81.3 ± 1.9 | 0.099 | 73.9 ± 2.3 | 0.141 |
| Incident newly diagnosed diabetes | 77.0 ± 2.2 | <0.001 | 78.6 ± 2.2 | 0.007 | 71.8 ± 2.4 | 0.027 |
| Bodily pain | ||||||
| NGT 1999 and 2005 | 76.8 ± 0.3 | Ref | 76.6 ± 0.3 | Ref | 75.6 ± 1.0 | Ref |
| Incident IFG | 69.9 ± 1.8 | <0.001 | 71.4 ± 1.8 | 0.005 | 67.3 ± 2.0 | 0.004 |
| Incident IGT | 71.6 ± 1.3 | <0.001 | 72.6 ± 1.3 | 0.004 | 68.8 ± 1.6 | 0.006 |
| Incident newly diagnosed diabetes | 70.3 ± 1.5 | <0.001 | 72.2 ± 1.5 | 0.005 | 68.8 ± 1.7 | 0.015 |
| General health | ||||||
| NGT 1999 and 2005 | 73.5 ± 0.3 | Ref | 73.3 ± 0.3 | Ref | 65.2 ± 0.8 | Ref |
| Incident IFG | 71.7 ± 1.5 | 0.234 | 73.2 ± 1.5 | 0.953 | 64.9 ± 1.6 | 0.819 |
| Incident IGT | 66.3 ± 1.2 | <0.001 | 67.4 ± 1.1 | <0.001 | 59.7 ± 1.3 | <0.001 |
| Incident newly diagnosed diabetes | 65.6 ± 1.5 | <0.001 | 68.8 ± 1.5 | <0.001 | 60.9 ± 1.4 | 0.001 |
| Vitality | ||||||
| NGT 1999 and 2005 | 64.4 ± 0.3 | Ref | 64.3 ± 0.3 | Ref | 59.7 ± 0.9 | Ref |
| Incident IFG | 62.8 ± 1.6 | 0.335 | 64.0 ± 1.6 | 0.881 | 59.3 ± 1.8 | 0.814 |
| Incident IGT | 60.4 ± 1.2 | 0.001 | 61.2 ± 1.2 | 0.012 | 56.8 ± 1.4 | 0.020 |
| Incident newly diagnosed diabetes | 59.4 ± 1.4 | <0.001 | 60.9 ± 1.4 | 0.017 | 57.1 ± 1.5 | 0.061 |
| Social functioning | ||||||
| NGT 1999 and 2005 | 89.5 ± 0.3 | Ref | 89.4 ± 0.3 | Ref | 86.1 ± 0.8 | Ref |
| Incident IFG | 87.0 ± 1.5 | 0.105 | 87.5 ± 1.5 | 0.229 | 84.1 ± 1.7 | 0.217 |
| Incident IGT | 87.0 ± 1.1 | 0.035 | 87.4 ± 1.1 | 0.078 | 84.1 ± 1.4 | 0.099 |
| Incident newly diagnosed diabetes | 83.8 ± 1.3 | <0.001 | 84.5 ± 1.3 | <0.001 | 81.5 ± 1.5 | 0.001 |
| Role-emotional | ||||||
| NGT 1999 and 2005 | 86.5 ± 0.5 | Ref | 86.5 ± 0.5 | Ref | 84.2 ± 1.4 | Ref |
| Incident IFG | 83.0 ± 2.5 | 0.153 | 83.8 ± 2.5 | 0.294 | 81.5 ± 2.8 | 0.280 |
| Incident IGT | 83.7 ± 1.8 | 0.128 | 84.3 ± 1.8 | 0.243 | 82.1 ± 2.2 | 0.261 |
| Incident newly diagnosed diabetes | 79.8 ± 2.1 | 0.001 | 80.8 ± 2.1 | 0.008 | 78.9 ± 2.4 | 0.014 |
| Mental health | ||||||
| NGT 1999 and 2005 | 77.3 ± 0.3 | Ref | 77.2 ± 0.3 | Ref | 75.8 ± 0.7 | Ref |
| Incident IFG | 75.7 ± 1.4 | 0.243 | 75.9 ± 1.4 | 0.356 | 74.4 ± 1.5 | 0.326 |
| Incident IGT | 74.2 ± 1.0 | 0.003 | 74.4 ± 1.0 | 0.007 | 73.1 ± 1.2 | 0.009 |
| Incident newly diagnosed diabetes | 74.3 ± 1.1 | 0.011 | 74.7 ± 1.1 | 0.028 | 73.5 ± 1.3 | 0.051 |
Data are marginal mean ± SEM.
*Model 1: SF-36 dimensions adjusted for diabetes status, age, and sex.
†Model 2: SF-36 dimensions adjusted for model 1 and BMI, systolic blood pressure and total cholesterol.
‡Model 3: SF-36 dimensions adjusted for model 2 and smoking, known cardiovascular disease and medication for hypertension or a lipid abnormality. Ref, referent.
Among those with incident IGT at follow-up, baseline scores for physical functioning, role-physical, bodily pain, general health, vitality, social functioning, and mental health were significantly lower compared with scores for those with NGT (after adjustment for age and sex). These differences in physical functioning, bodily pain, general health, vitality, and mental health were only partially attenuated by adjustment for systolic blood pressure, BMI, and total cholesterol. Further adjustment for known CVD, smoking, lipid abnormality, and hypertension reduced the mean values of each dimension but did not significantly alter the association. Among those with incident IFG at follow-up, baseline scores for Physical Functioning, Role-Physical, and Bodily Pain were significantly lower compared with scores for those with NGT. However, after further adjustment for BMI, systolic blood pressure, and total cholesterol at baseline only the difference for Bodily Pain remained. Further adjustment for known CVD, smoking, lipid abnormality, and hypertension reduced the mean values of each dimension but did not significantly alter the association.
Further inclusion of baseline FPG in a fifth model failed to attenuate the associations observed for each dimension of the SF-36 (data not shown). A final model comparing those with and without incident diabetes (using all available data, n = 5,450) made no difference to the association of diabetes with impaired quality of life on the physical functioning, bodily pain, general health, social functioning, and role-emotional dimensions (after adjustment for age, sex, BMI, total cholesterol, smoking, known CVD, and medication for hypertension or a lipid abnormality. Results were as follows (mean ± SEM): for physical functioning, no diabetes 78.5 ± 0.6 and diabetes 74.9 ± 1.2 (P = 0.002); for role-physical, no diabetes 75.9 ± 1.1 and diabetes 71.9 ± 2.4 (P = 0.070); for bodily pain, no diabetes 71.6 ± 0.8 and diabetes 68.4 ± 1.6 (P = 0.043); for general health, no diabetes 64.7 ± 0.6 and diabetes 61.2 ± 1.3 (P = 0.004); for vitality, no diabetes 59.3 ± 0.7 and diabetes 57.0 ± 1.5 (P = 0.104); for social functioning, no diabetes 84.6 ± 0.7 and diabetes 81.0 ± 1.4 (P = 0.006); for role-emotional no diabetes 82.1 ± 1.1 and diabetes 77.9 ± 2.3 (P = 0.051); and for mental health, no diabetes 74.8 ± 0.6 and diabetes 72.8 ± 1.2 (P = 0.096).
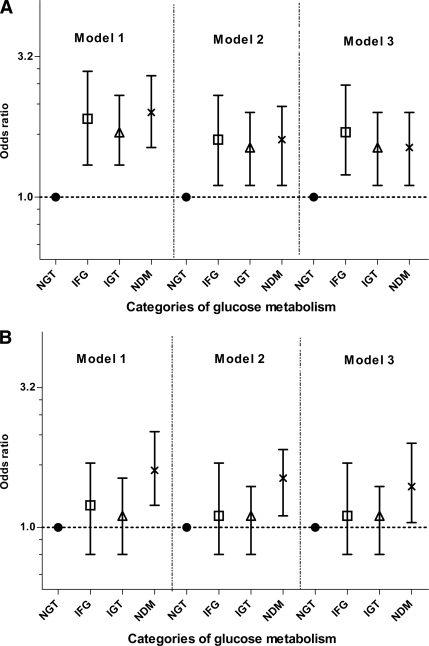
The physical functioning summary score showed a significant gradual decrease across categories of glucose metabolism from NGT through to incident diabetes (Fig. 1). After we controlled for factors associated with incident diabetes, those in the lowest quartile of the PCS scale at baseline had at least a 50% higher risk of progression to impaired glucose metabolism and diabetes 5 years later (IFG odds ratio 1.7 [95% CI 1.2–2.5], IGT 1.5 [1.1–2.1], and newly diagnosed diabetes 1.5 [1.1–2.1]). An association only with NDM was evident for the MCS measure.
Figure 1.
A: PCS scale of AusDiab study participants according to diabetes status at the 5-year follow-up. B: MCS scale of AusDiab study participants according to diabetes status at the 5-year follow-up. Error bars represent 95% CI. Model 1 was adjusted for age and sex; model 2 was adjusted for age, sex, BMI, systolic blood pressure, and total cholesterol; and model 3 was adjusted for age, sex, BMI, systolic blood pressure, total cholesterol, current treatment for hypertension or lipid abnormalities, and known CVD. The PCS is a summary of physical functioning, role-physical, bodily pain, and general health. The MCS is a summary measure of vitality, social functioning, role-emotional, and mental health. NDM, newly diagnosed diabetes.
CONCLUSIONS
There were three principal findings in this national, population-based study from Australia. First, those with incident IFG had increased bodily pain before the onset of IFG (model 2). Second, those with IGT had increased bodily pain and reduced physical functioning, general health, vitality, and mental health before the onset of IGT. Third, those with incident newly diagnosed diabetes had impaired HRF on each dimension of the SF-36 before the onset of type 2 diabetes. These are important findings. The impact of HRF on subsequent development of incident impaired glucose metabolism and type 2 diabetes, as far as we are aware, has not previously been explored in a large national representative population-based sample. The identification of impaired HRF factors before the onset of impaired glucose metabolism and newly diagnosed diabetes suggests that HRF is likely to be important in the identification of individuals at high risk of type 2 diabetes.
Our study results can be compared with the few population-based studies available (3,4). In a cross-sectional Australian study of 4,060 individuals, those with IFG had significantly worse scores for physical functioning and bodily pain than those with NGT (after adjustment for age, sex, and cardiovascular disease) (4). Although the AusDiab cross-sectional study showed no impairment in HRF among those with IFG compared with those with NGT, those with IGT had impaired physical and social functioning compared with those with NGT (after adjustment for age, sex, BMI, physical activity, and treatment for hypertension or a lipid abnormality) (5). In a study by Hiltunen et al. (3), a pattern of general worsening of HRF across categories of glucose tolerance status was evident. However, because of the limited sample size, no firm conclusions could be drawn. In the present study, those with incident newly diagnosed diabetes had worse scores on all SF-36 dimensions apart from mental health and vitality compared with those with NGT (model 3). This result is very similar to the findings of the study by Chittleborough et al. (4), who showed that HRF was impaired on all dimensions of the HRF scale compared with those with NGT.
A consistent finding through the limited number of studies to assess HRF across the spectrum of glycemic values is that the decline in HRF is related to the physical component of the SF-36, with mixed results shown for the mental component (3–5). A similar pattern has been observed in research focused on HRF with adiposity, coronary heart disease, and insulin resistance (18–22). It is likely that inactivity is a risk factor for both impaired HRF and impaired glucose metabolism and type 2 diabetes. In terms of physical functioning, metabolic abnormalities underpinning type 2 diabetes have been associated with reduced muscle strength and impaired HRF in the domain of physical functioning. In a large prospective study assessing body weight and HRF, the study showed that after 4 years, weight gain was associated with decreased physical functioning and vitality and increased bodily pain, regardless of baseline weight status (23). In contrast, those who lost weight had increased physical functioning and vitality and decreased bodily pain. Identification of individuals with mild to moderately impaired physical functioning could provide some prognostic information on the future risk of diabetes very early in the disease process. This finding is particularly relevant as it is well established that there is a strong link between physical inactivity, obesity, and type 2 diabetes. Given that improved HRF among those with diabetes is known to improve self-management and lead to lifestyle changes, crucial for improved glycemic control, HRF across the continuum of glycemic values requires further investigation (24).
The findings from this study raise questions about the casual pathways and mechanisms involved in diabetes onset and its progression related to HRF and other salient psychosocial determinants. For example, the presence of reduced HRF in the years preceding disease onset may suggest that the process is mediated through the behavioral pathways known to reduce HRF and act as risk factors for chronic disease (e.g., increased sedentary behavior as a result of reduced functioning). Although data from epidemiological cohort studies have previously refuted the suggestion that associations between psychosocial factors and disease risk are likely to be mediated through behavioral pathways (25), further research is required into the psychosocial determinants of chronic disease onset to provide a clearer picture of the associated casual pathways.
The current study has limitations. The follow-up response rate for the AusDiab study was limited, and this may have led to an underestimation of the association between quality-of-life measures and glucose metabolism. The results of this study suggest there is an independent association between impaired HRF and IFG, IGT, and NDM. It should be noted that this association is likely to be bidirectional. For example, among those with known diabetes, the presence of complications is associated with a reduced HRF and the greater the number or severity of complications, the greater the decrease in HRF. This could be a consequence of having a number of complications decreasing quality of life or conversely the impact of poor HRF on diabetes control.
In summary, these findings show that IFG, IGT, and newly diagnosed diabetes are associated with reduced HRF and that this is evident before the onset of these conditions, independent of known CVD. This is an important finding. If future health promotion campaigns are to effectively target those at high risk of developing diabetes, an understanding of the process of declining health before the onset of the disease is essential.
Acknowledgments
The AusDiab study, co-coordinated by the Baker IDI Heart and Diabetes Institute, gratefully acknowledges the generous support given by National Health and Medical Research Council (Grant 233200), Australian Government Department of Health and Ageing, Abbott Australasia Pty, Alphapharm Pty, AstraZeneca, Aventis Pharma, Bristol-Myers Squibb, City Health Centre-Diabetes Service–Canberra, Department of Health and Community Services–Northern Territory, Department of Health and Human Services–Tasmania, Department of Health–New South Wales, Department of Health–Western Australia, Department of Health–South Australia, Department of Human Services–Victoria, Diabetes Australia, Diabetes Australia Northern Territory, Eli Lilly Australia, Estate of the Late Edward Wilson, GlaxoSmithKline, Jack Brockhoff Foundation, Janssen-Cilag, Kidney Health Australia, Marian & FH Flack Trust, Menzies Research Institute, Merck Sharp & Dohme, Novartis Pharmaceuticals, Novo Nordisk Pharmaceuticals, Pfizer Pty, Pratt Foundation, Queensland Health, Roche Diagnostics Australia, Royal Prince Alfred Hospital, Sydney, and Sanofi Synthelabo. R.J.T. is supported by a Sidney Sax fellowship from the National Health and Medical Research Council of Australia (Grant 334173).
No other potential conflicts of interest relevant to this article were reported.
For their invaluable contribution to the setup and field activities of AusDiab, we thank A. Allman, B. Atkins, S. Bennett, A. Bonney, S. Chadban, M. de Courten, M. Dalton, D. Dunstan, T. Dwyer, H. Jahangir, D. Jolley, D. McCarty, A. Meehan, N. Meinig, S. Murray, K. O'Dea, K. Polkinghorne, P. Phillips, C. Reid, A. Stewart, H. Taylor, T. Whalen, and F. Wilson.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Commonwealth of Australia. National Diabetes Strategy 2000–2004. Canberra, Commonwealth Department of Health and Aged Care, 1999, p. 1–16 [Google Scholar]
- 2. Toshihiro M, Saito K, Takikawa S, Takebe N, Onoda T, Satoh J. Psychosocial factors are independent risk factors for the development of type 2 diabetes in Japanese workers with impaired fasting glucose and/or impaired glucose tolerance. Diabet Med 2008;25:1211–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hiltunen L, Keinänen-Kiukaanniemi S. Does glucose tolerance affect quality of life in an elderly population? Diabetes Res Clin Pract 1999;46:161–167 [DOI] [PubMed] [Google Scholar]
- 4. Chittleborough CR, Baldock KL, Taylor AW, Phillips PJ. North West Adelaide Health Study Team. Health status assessed by the SF-36 along the diabetes continuum in an Australian population. Qual Life Res 2006;15:687–694 [DOI] [PubMed] [Google Scholar]
- 5. Tapp RJ, Dunstan DW, Phillips P, Tonkin A, Zimmet PZ, Shaw JE. AusDiab Study Group. Association between impaired glucose metabolism and quality of life: results from the Australian diabetes obesity and lifestyle study. Diabetes Res Clin Pract 2006;74:154–161 [DOI] [PubMed] [Google Scholar]
- 6. Quality of life in type 2 diabetic patients is affected by complications but not by intensive policies to improve blood glucose or blood pressure control (UKPDS 37). Diabetes Care 1999;22:1125–1136 [DOI] [PubMed] [Google Scholar]
- 7. Lloyd A, Sawyer W, Hopkinson P. Impact of long-term complications on quality of life in patients with type 2 diabetes not using insulin. Value Health 2001;4:392–400 [DOI] [PubMed] [Google Scholar]
- 8. De Rekeneire N, Resnick HE, Schwartz AV, Shorr RI, Kuller LH, Simonsick EM, Vellas B, Harris TB. Diabetes is associated with subclinical functional limitation in nondisabled older individuals: the Health, Aging, and Body Composition study. Diabetes Care 2003;26:3257–3263 [DOI] [PubMed] [Google Scholar]
- 9. Manuel DG, Schultz SE. Health-related quality of life and health-adjusted life expectancy of people with diabetes in Ontario, Canada, 1996–1997. Diabetes Care 2004;27:407–414 [DOI] [PubMed] [Google Scholar]
- 10. Black PH, Garbutt LD. Stress, inflammation and cardiovascular disease. J Psychosom Res 2002;52:1–23 [DOI] [PubMed] [Google Scholar]
- 11. Black PH. The inflammatory consequences of psychologic stress: relationship to insulin resistance, obesity, atherosclerosis and diabetes mellitus, type II. Med Hypotheses 2006;67:879–891 [DOI] [PubMed] [Google Scholar]
- 12. Blumenthal JA, Babyak M, Wei J, O'Connor C, Waugh R, Eisenstein E, Mark D, Sherwood A, Woodley PS, Irwin RJ, Reed G. Usefulness of psychosocial treatment of mental stress-induced myocardial ischemia in men. Am J Cardiol 2002;89:164–168 [DOI] [PubMed] [Google Scholar]
- 13. Dunstan D, Zimmet P, Welborn T, Cameron A, Shaw J, deCourten M, Jolley D, McCarty D. AusDiab Steering Committee. The Australian Diabetes, Obesity and Lifestyle Study (AusDiab)-methods and response rates. Diabetes Res Clin Pract 2002;57:119–129 [DOI] [PubMed] [Google Scholar]
- 14. Magliano DJ, Barr EL, Zimmet PZ, Cameron AJ, Dunstan DW, Colagiuri S, Jolley D, Owen N, Phillips P, Tapp RJ, Welborn TA, Shaw JE. Glucose indices, health behaviors and incidence of diabetes in Australia: the Australian Diabetes, Obesity and Study. Diabetes Care 2007;31:267–272 [DOI] [PubMed] [Google Scholar]
- 15. World Health Organization. Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications. Part 1: Diagnosis and Classification of Diabetes Mellitus. Geneva, Department of Noncommunicable Disease Surveillance, 1999. [Google Scholar]
- 16. Briganti EM, Shaw JE, Chadban SJ, Zimmet PZ, Welborn TA, McNeil JJ, Atkins RC. Untreated hypertension among Australian adults: the 1999–2000 Australian Diabetes, Obesity and Lifestyle Study (AusDiab). Med J Aust 2003;179:135–139 [DOI] [PubMed] [Google Scholar]
- 17. Ware J, Kosinski M, Keller S. SF-36 Physical and Mental Health Summary Scales: A User's Manual. Boston, The Health Institute, 1994. [Google Scholar]
- 18. Finkelstein MM. Body mass index and quality of life in a survey of primary care patients. J Fam Pract 2000;49:734–737 [PubMed] [Google Scholar]
- 19. Fekkes M, Kamphuis RP, Ottenkamp J, Verrips E, Vogels T, Kamphuis M, Verloove-vanhorick P. Health-related quality of life in young adults with minor congenital heart disease. Psychol Health 2001;16:239–250 [Google Scholar]
- 20. Fontaine KR, Barofsky I. Obesity and health-related quality of life. Obes Rev 2001;2:173–182 [DOI] [PubMed] [Google Scholar]
- 21. He XZ, Baker DW. Body mass index, physical activity, and the risk of decline in overall health and physical functioning in late middle age. Am J Public Health 2004;94:1567–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schlotz W, Ambery P, Syddall HE, Crozier SR, Sayer AA, Cooper C, Phillips DI. Hertfordshire Cohort Study Group. Specific associations of insulin resistance with impaired health-related quality of life in the Hertfordshire Cohort Study. Qual Life Res 2007;16:429–436 [DOI] [PubMed] [Google Scholar]
- 23. Fine JT, Colditz GA, Coakley EH, Moseley G, Manson JE, Willett WC, Kawachi I. A prospective study of weight change and health-related quality of life in women. JAMA 1999;282:2136–2142 [DOI] [PubMed] [Google Scholar]
- 24. Zhang X, Norris SL, Chowdhury FM, Gregg EW, Zhang P. The effects of interventions on health-related quality of life among persons with diabetes: a systematic review. Med Care 2007;45:820–834 [DOI] [PubMed] [Google Scholar]
- 25. Steptoe A, Marmot M. Burden of psychosocial adversity and vulnerability in middle age: associations with biobehavioral risk factors and quality of life. Psychosom Med 2003;65:1029–1037 [DOI] [PubMed] [Google Scholar]