Figure 2. Schematic illustration of adenovirus vector immobilization on the metal surface of stents.
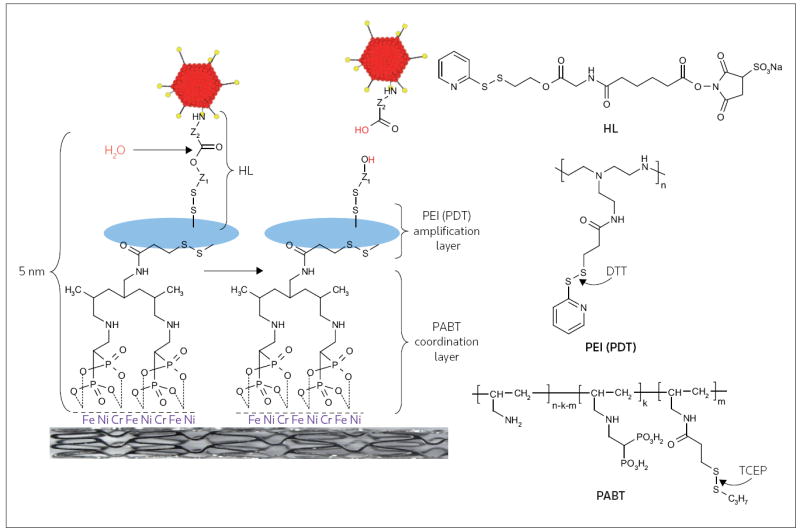
Serotype 5 adenovirus (Ad) vectors were modified by reacting the lysine residues of the viral capsid proteins with a reactive bifunctional amine/thiol hydrolyzable crosslinker (HL) that possessed a hydrolyzable ester bond separating fragments Z1 and Z2. Stainless steel stents were consecutively exposed to a solution of polyallylamine bisphosphonate comprising latent thiol groups (PABT) and the reducing agent tris(2-carboxyethyl)phosphine (TCEP) to activate thiol groups on the PABT-coated surface. To expand the quantity of available thiol functional groups, the stent was also treated with polyethyleneimine modified with pyridyldithio-groups (PEI(PDT)) and dithiothreitol (DTT). Finally, HL-modified Ad vectors were reacted with the thiolated metal surfaces, leading to the covalent tethering of Ad on the stent surface. The release of the covalently immobilized Ad was dependent on the rate of hydrolysis of the ester bond in the HL backbone.
(Adapted from the American Heart Association Inc and Fishbein I, Alferiev I, Bakay M, Stachelek SJ, Sobolewski P, Lai M, Choi H, Chen IW, Levy RJ: Local delivery of gene vectors from bare-metal stents by use of a biodegradable synthetic complex inhibits in-stent restenosis in rat carotid arteries. Circulation (2008) 117(16):2096-2103 © 2008 American Heart Association Inc)
