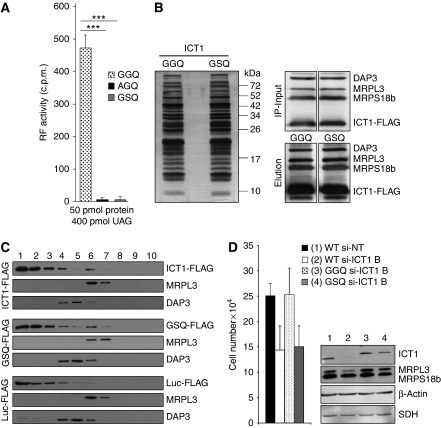
Figure 4.
Mutations of the GGQ domain can affect cell viability. (A) GGQ-mutant derivatives of ICT1 have lost PTH activity. Wild type and mutant derivatives (AGQ, GSQ) of Δ29 ICT1 were expressed as GST-fusion proteins, cleaved and assayed for f[3H]met release as described in Figure 3. All assays were performed with UAG codons and purified proteins, all equally monodispersed as assessed by dynamic light scattering (data not shown); ***P<0.001. (B, C) Mutated ICT1 is assembled into the mitoribosome. (B) FLAG-tagged wild-type (GGQ) and mutant (GSQ) ICT1 were expressed in HEK293T cells and the eluate from FLAG IP was subjected to silver staining (left panel) or western blotting (right panels) after denaturing gel electrophoresis. Molecular weight markers are indicated. The western blots of mitochondrial lysates shown are those before (IP-input) and after (Elution) FLAG IP of the wild type and mutated ICT1 derivatives. (C) Cell lysates were subjected to isokinetic gradient analysis before fractionation and western blotting, as described. The upper three panels are from wild-type ICT1-FLAG, the middle panels from mutated GSQ ICT1-FLAG and the lower panels from control mtLuc-FLAG. (D) A mutation of the GGQ domain affects cell growth. Non-targeting si-RNA-treated cells served as negative control (1, WT si-NT). Cells with only endogenous ICT1 (WT), or overexpressing normal (GGQ) or mutated (GSQ) ICT1 were treated for 4 days with 10 nM si-ICT1B to deplete endogenous ICT1 (2–4), whereas lane-2 represented the fully depleted control (WT si-ICT1B). Growth rates were compared by counting populations after 3 days of siRNA treatment; 3 versus 4: P<0.01; 1 versus 4: P<0.001. Western blots of lysates (4 days of siRNA treatment) after interrogation with the indicated antibodies are shown to the right.