Figure 5.
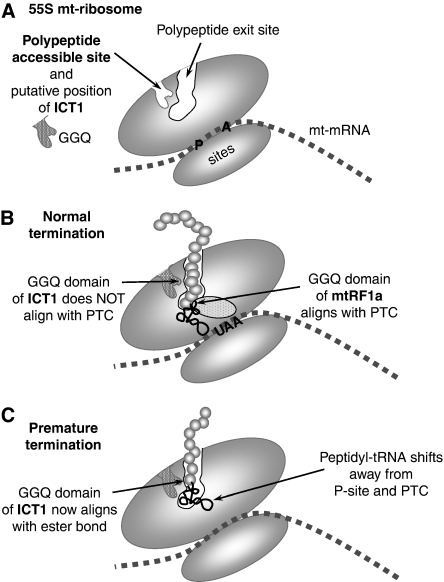
A schematic representation of the putative position and function of ICT1 in the human mitochondrial ribosome. (A) A simplified cartoon of the 55S mitochondrial ribosome indicating the polypeptide exit tunnel and site (PES) and the PAS in the large subunit as defined by Sharma et al (2003). No orthologues of the proteins that would occupy the PAS have been found in mammalian mitochondria, and we postulate that is where ICT1 is positioned with the GGQ domain inserted deep into the pocket. Sites for the aminoacyl (A) and peptidyl (P) tRNAs are shown. The mt-mRNA is depicted between the large and small mt-ribosomal subunits. (B) Under conditions of normal termination, the ester bond of the peptidyl-tRNA is positioned close to the peptidyl-transferase centre (PTC); the release factor, mtRF1a, enters through the A-site, recognising the stop codon (UAA) and aligning the GGQ domain at the PTC to promote hydrolysis of the ester bond and release of the nascent peptide. (C) Where abortive elongation occurs, the peptidyl-tRNA may drop away from the P-site towards the PES, aligning the ester bond close to the GGQ domain of ICT1, promoting cleavage of the tRNA, which allows both mt-tRNA and truncated peptide to be released from the mitochondrial monosome (or potentially from dissociated 39S mt-LSU).