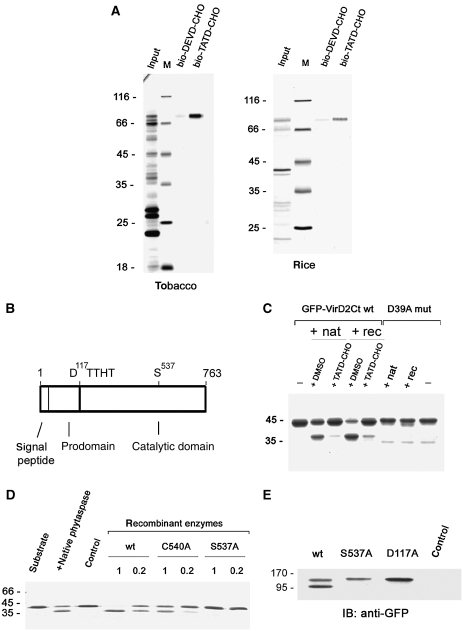
Figure 1.
Identification and molecular characterisation of tobacco phytaspase. (A) Affinity chromatography purification of tobacco (left panel) and rice (right panel) phytaspases with their inhibitor, bio-TATD-CHO on avidin resin; bio-DEVD-CHO, which does not inhibit phytaspase, was used as a control. Protein samples were fractionated by SDS–gel electrophoresis and stained with Coomassie Blue or zinc-imidazole. Positions of MW protein markers (M) are indicated on the left. (B) Schematic representation of phytaspase domains. (C) Recombinant tobacco phytaspase (rec) displays the same ability to cleave GFP-VirD2Ct protein as natural tobacco enzyme (nat). The bio-TATD-CHO was used at 100 μM for enzyme inhibition. Coomassie Blue-stained gel is shown. The D39A mutation (D39A mut) (Chichkova et al, 2004) preventing cleavage in the GFP-VirD2Ct protein corresponds to the D400 residue in full-length VirD2. (D) The S537A mutation prevents phytaspase-mediated cleavage of GFP-VirD2Ct in vitro (Coomassie Blue staining). C540A, a mutation of a nearby residue, only marginally affects proteolytic activity. Recombinant enzymes were produced as in (C). 1 and 0.2 indicate relative amounts of recombinant enzymes taken to assess their hydrolytic activity, as verified by western blotting with anti-GST antibody. Control sample (control) obtained in an identical manner from vector-only agroinfiltrated leaves. (E) Phytaspase mutants with impaired processing. Western blot analysis of the crude extracts from leaves agroinfiltrated with constructs expressing wt and mutated phytaspase forms fused to GFP with anti-GFP antibody. In contrast to the purified enzyme (A) showing only one band (mature enzyme), extracts containing wt phytaspase display two bands (proenzyme and mature enzyme) apparently because in the latter case, we used immediate denaturation of samples (boiling in SDS). Both the S537A (catalytic residue) and D117A (the prodomain/catalytic domain junction) mutations impair proenzyme processing in N. benthamiana leaves. Control, vector-only agroinfiltrated leaves.