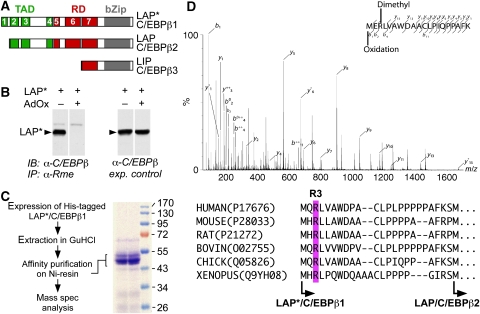
Figure 1.
C/EBPβ is post-translationally methylated on arginine residues. (A) Scheme of C/EBPβ isoforms that arise by alternative translation initiation from in-frame start codons termed as LAP*/C/EBPβ1, LAP/C/EBPβ2 and LIP/C/EBPβ3. (B) LAP*/C/EBPβ1 is precipitated by the ASYM24 anti-Rme2a antibody in the absence of AdOx. LAP*/C/EBPβ1-transfected fibroblasts were treated with adenosine dialdehyde (AdOx) for 12 h (as indicated) and immunoprecipitated with the ASYM24 antibody. Precipitated proteins were analysed by immunoblotting using an anti-C/EBPβ antibody. Right side shows expression controls in the absence and presence of AdOx. (C) Purification scheme of C/EBPβ. Briefly, His-tagged C/EBPβ was purified under denaturing conditions by affinity chromatography on a nickel chelating resin. Blot shows purified fractions of C/EBPβ. (D) Tandem mass spectrum of an R3me2 and oxidized N-terminal C/EBPβ peptide. b- and y-series of the LysC-generated peptides are indicated. Mass shifts indicate a 28 Da modification, corresponding to dimethylation at position R3. Underneath: alignment of conserved region 1 (CR1) of LAP*/C/EBPβ1 from various species shows conserved R3 (magenta box) and start sites (arrows) of the two long isoforms.