Table 2.
Ru-catalyzed reductive coupling of aldehydes 3a-3f to 1,1-disubstituted allenes via transfer hydrogenation.a
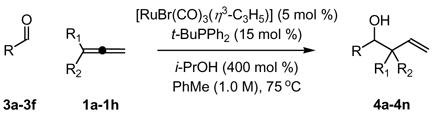 | |||
|---|---|---|---|
| 3a, R = p-NO2Ph | 3c, R = 2-(4-NO2-Furyl) | 3e, R = CH2OBn | |
| 3b, R = p-(CO2Me)Ph | 3d, R = o-NO2-Cinnamyl | 3f, R = CH2NPhth | |
| entry | RCHO, allene | product | yield % (dr) |
| 1 | 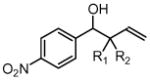 |
||
| 3a, 1a | 4a, R1 = Me, R2 = Ph | 87% (2:1 dr) | |
| 3a, 1b | 4b, R1 = Me, R2 = p-FPh | 86% (2:1 dr) | |
| 3a, 1d |
4c, R1 = CH2OMe R2 = p-MeOPh |
76% (1:1 dr) | |
| 3a, 1f | 4d, R1 = Me, R2 = CH2Ph | 83% (1:1 dr) | |
| 3a, 1g | 4e, R1 = Et, R2 = n-Bu | 82% (1:1 dr) | |
| 2 | 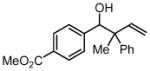 |
||
| 3b, 1a | 4f | 69% (2:1 dr) | |
| 3 | 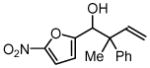 |
||
| 3c, 1a | 4g | 74% (1:1 dr) | |
| 4 | 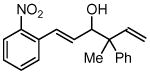 |
||
| 3d, 1a | 4h | 61% (2:1 dr) | |
| 5 | 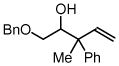 |
||
| 3e, 1a | 4i, R1= Me, R2 = Ph | 71% (1:1 dr) | |
| 3e, 1d |
4j, R1= CH2OMe R2 = p-MeOPh |
72% (1:1 dr) | |
| 3e, 1g | 4k, R1 = Et, R2 = n-Bu | 70% (1:1 dr) | |
| 6 | 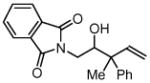 |
||
| 3f, 1a | 4l, R1= Me, R2 = Ph | 76% (1:1 dr) | |
| 3f, 1b | 4m, R1 = Me, R2 = p-FPh | 77% (1:1 dr) | |
| 3f, 1g | 4n, R1 = Et, R2 = n-Bu | 74% (1:1 dr) | |
Cited yields are of pure isolated material. See Supporting Information for detailed experimental procedures.
