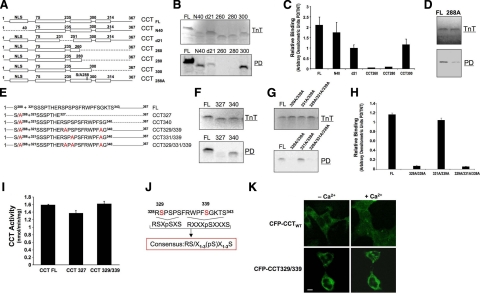
Figure 4.
Mapping of a 14-3-3ζ-binding site within CCTα. A) Map illustrates sequences of wild-type or individual CCTα mutants using deletional mutagenesis. Dashed lines represent deleted residues. Constructs tested include wild-type CCT (CCTFL), an NH2-terminal deletion mutant (CCTN40) devoid of the nuclear localization signal (NLS), and an internal deletion mutant lacking 21 residues within the catalytic-membrane binding hinge region (CCTd21). Other constructs include a series of deletion mutants progressively truncated within the CCTα carboxyl terminus (CCT260, CCT280, and CCT300) or point mutations (S288). B) TnT: individual mutants were synthesized in vitro using rabbit reticulocyte lysate in a reaction containing [35S]-methionine (20 μCi/reaction). Pulldown (PD): newly translated reaction products were incubated with 14-3-3ζ beads and sedimented, and pellets were processed for SDS-PAGE and autoradiography. C) Relative binding of constructs as shown by densitometry and expressed as ratios of synthesized/bound protein levels. D) Full-length CCTα construct harboring a point mutation at S288 was tested for 14-3-3ζ binding. E) Map illustrates sequences of wild-type CCTα (CCTFL), or 5 individual CCTα mutants all containing a Ser-288A point mutation with additional deletions or multiple Ser to Ala substitutions within the phosphorylation domain. Dashed lines represent deleted residues. CCT327 and CCT340 are carboxyl-terminal truncated variants (containing 327 and 340 residues, respectively). Remaining CCTα mutants are full-length constructs all harboring multiple-point mutations as candidate phosphoserine sites. F) Individual CCT327 and CCT340 mutants were synthesized in vitro using TnT as above; products were incubated with 14-3-3ζ beads and processed for SDS-PAGE and autoradiography as in B. G, H) Binding of phosphomimetic mutation constructs as shown by autoradiography (G) and densitometry (H). I) CCT activities of TnT-synthesized mutants, CCT327 and CCT329/339, that lacked ability to bind 14-3-3, were determined to assess folding of mutants. J) Sequence alignment. CCTα residues 328–343 are at top, the known consensus 14-3-3 binding motifs are at center (underscored), and a proposed modified motif for 14-3-3ζ binding as identified within CCTα by mapping is at bottom. K) MLE cells were transfected with CFP-CCTα or CFP-CCT329/339 plasmids in the presence or absence of Ca2+ as in Fig. 1A. Cells were then visualized by confocal microscopy for subcellular localization. Compared to nuclear and cytosolic CFP-CCTα, CFP-CCT329/339 was retained within the cytoplasm despite an exogenous Ca2+ stimulus. Scale bar = 10 μm. Data in immunoblot panels represent n = 3 separate experiments (except CCTN40 construct; n=2). Densitometric data represent means ± sd.