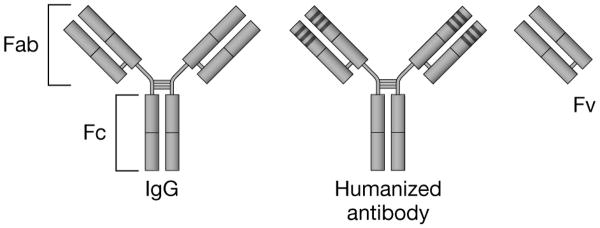
Figure 2. Structure of immunoglobin and monoclonal antibody fragments.
The immunoglobulin-G (IgG) molecule is composed of two longer (heavy) chains and two shorter (light) chains that are connected by disulfide bonds. The amino acid sequence of the variable (v) ends of each of the four chains determines the specificity of antigen binding. Humanized monoclonal antibody constructs consist predominantly of human amino acid sequences, with the exception of the three hypervariable complementarity-determining regions (CDR), which retain the foreign-specie sequences critical for antigen binding. The CDR domains are indicated by the dark bands at the end of the antigen-binding portion of the molecule.
Fab: Antigen binding fragment; Fc: Crystallizable or complement fixing fragment; Fv: Variable fragment