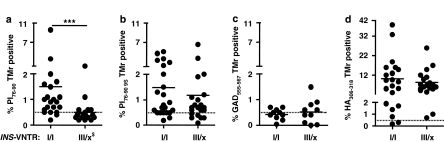
Figure 1.
Relationship between INS-VNTR genotype and detection of CD4 T cells, which bind PI76–90 tetramers. Subjects with VNTR class I alleles have high levels of peripheral blood T cells recognizing soluble DRB1*0401-PI76–90 tetramer compared with the subjects having the protective class III alleles (a). The same T-cell samples were analyzed using the PI76–90 9S modified tetramer; no difference between the two groups of subjects was detected, implying presence of low-avidity PI-reactive T cells in both (b). In addition, no difference in tetramer positivity between the two groups of subjects was found when tetramers specific for another T1D autoantigen, GAD555–567, or foreign antigen, HA306–318, were used, indicating proinsulin specificity of the VNTR association (c, d). ***P=0.0004, differences in the level of tetramer-reactive T cells were calculated using Mann–Whitney U test. Solid horizontal lines represent means. Dashed horizontal lines indicate 0.5% binding of negative control tetramer. $INS-VNTR III/x where x=I or III. Typing for HLA-DRB1*04 subtypes was performed using sequence-specific primers and probes in conjunction with real-time PCR.27 A total of 45 HLA-DRB1*0401-positive subjects were analyzed out of which 6 were homozygous for DRB1*0401, 12 had in addition DRB1*0301 and the remaining 27 had in addition some other DRB1* allele. T1D patients of INS-VNTR I/I as well as INS-VNTR I/III genotype were of comparable age (average 25.8±8.9 years vs 26.2±11.6 years, respectively) and had similar duration of the disease (3.4±1.9 years vs 5.5±1.5 years, respectively) as were autoantibody-positive subjects (36.3±10.4 vs 42±12.8) and healthy controls (40.4±14.5 vs 50.5±14.6). The VNTR genotype was assigned based on the genotype at the −23 A/T single-nucleotide polymorphism (SNP) at the INS promoter.14, 28 Peripheral blood mononuclear cells were isolated from heparinized blood and CD4+CD25− T cells enriched in two steps. In the first step CD4+ T cells were separated from all non-CD4 cells using CD4+ T cell isolation kit (Miltenyi Biotec, Auburn, CA, USA). In the second step CD25+ T cells were depleted using anti-CD25-coated micro beads. CD4+CD25− T cells were stimulated in vitro for 12–14 days in 48-well tissue culture plates with autologous adherent non-CD4+ cells prepulsed with either PI peptides, hemagglutinin (HA) peptide (10 μg ml−1) or without peptides. At days 6, 8 and 10, human recombinant IL-2 (10 IU ml−1, Chiron Corporation, Emeryville, CA, USA) was added to the cultures.