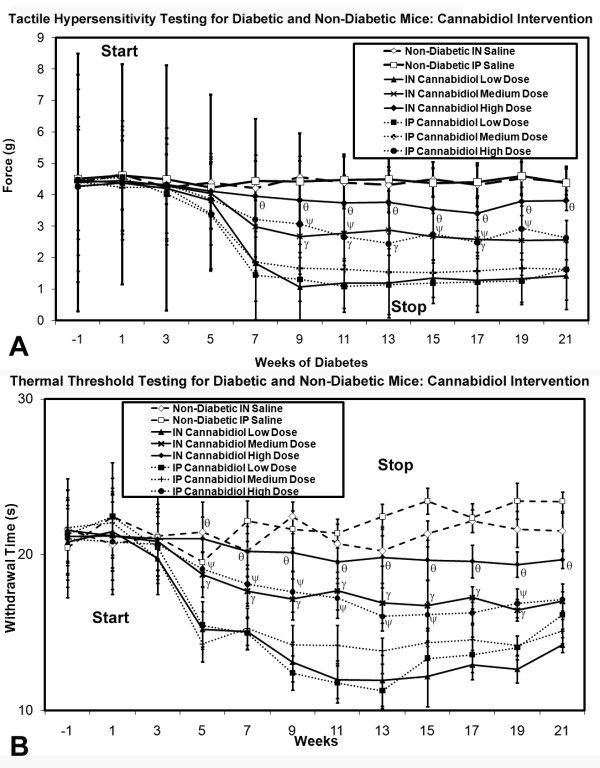
Figure 5.
Tactile (A) and thermal (B) sensory testing data for diabetic mice receiving either intranasal or intraperitoneal cannabidiol at low, medium, or high dose, with comparison to non-diabetic mice and saline delivery. Diabetic mice receiving medium or high doses of intranasal or intraperitoneal cannabidiol had amelioration of both tactile allodynia and thermal hyperalgesia after 7 weeks when nociceptive behaviors began. This protection against the development of the neuropathic pain state was also noted continually after the stoppage of cannabidiol at week 14. For both tactile (A) and thermal (B) testing, significant differences were detected between the diabetic mouse group receiving medium (γ) or high doses (θ) of intranasal cannabidiol or high (Ψ) doses of intraperiteonal cannabidiol when compared to the diabetic mouse group receiving low dose intranasal or intraperitoneal cannabidiol respectively (non-matched ANOVA tests, F-values range between 0.88-4.13 for indicated groups and time points, n ≥ 4, p < 0.05). Area under the curve (AUC) measurements were also significantly different between the same comparison groups in each case (p < 0.05). [n = 4-10 mice in each mouse cohort for each time point]