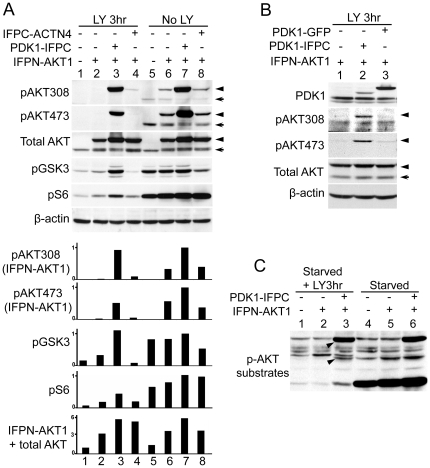
Figure 2. PI3K-independent AKT phosphorylation and activation.
(A) AKT phosphorylation and activation in the PDK1-IFPC::IFPN-AKT1 complex. Four stable cell lines, parental HeLa, cells expressing IFPN-AKT1 only, cells co-expressing IFPN-AKT1 and PDK1-IFPC, and cells co-expressing IFPN-AKT1 and IFPC-ACTN4, were serum starved for overnight then treated or not treated with LY294002 (20 µM) for 3 hours. Cells were lysed in RIPA buffer supplied with protease inhibitors and phosphatase inhibitors. Lysates (50 µg/lane) were resolved in 10% SDS PAGE. Antibodies for each blot were listed to the left of the blots. β-actin immunoblotting shows equivalent loading. The arrow head designates IFPN-AKT1. The arrow designates endogenous AKT. Scanning densitometric values of western blots were obtained using the NIH image 1.63.1 software. IFPN-AKT1 phosphorylation was normalized to total IFPN-AKT1. GSK3 (S21/9) and S6 (S235/236) phosphorylation was normalized to β-actin. Data were presented as relative conversion to values of the sample in lane 7. (B) The reconstituted IFP is indispensable for the PI3K-independent IFPN-AKT1 phosphorylation. HeLa cells stably expressing IFPN-AKT1 were transiently transfected with PDK1-IFPC or PDK1-GFP. Cells were serum starved for overnight and then treated with LY294002 (20 µM) for 3 hours. The arrow head designates IFPN-AKT1. The arrow designates endogenous AKT. (C) Phosphorylation of AKT substrates by the PDK1-IFPC::IFPN-AKT1 complex. Parental HeLa, cells stably expressing IFPN-AKT1 only, and cells stably co-expressing IFPN-AKT1 and PDK1-IFPC were serum starved for overnight then treated or not treated with LY294002 (20 µM) for 3 hours. Anti-phospho-AKT substrate antibody was used to detect the phosphorylation of AKT substrates. Two arrow heads designate the protein species with elevated phosphorylation level in the LY294002 treated cells stably expressing the PDK1-IFPC::IFPN-AKT1 complex.