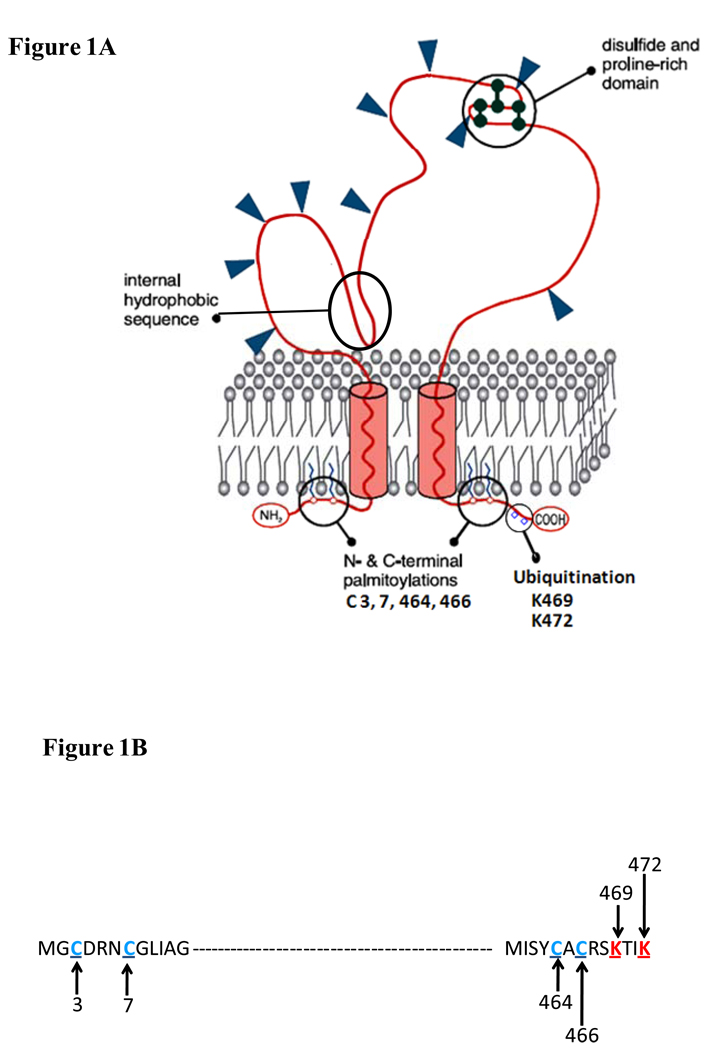
Figure 1. Predicted topography of CD36 in the plasma membrane.
(a) CD36 is a heavily glycosylated protein of 471 amino acids. Glysosylation accounts for the difference between the apparent (88Kd) and predicted (~53Kd) molecular weight of the protein. CD36 has a hairpin configuration in the plasma membrane. The extracellular domain has multiple N-linked glycosylation sites, a proline rich domain and a hydrophobic stretch that may associate with the membrane. The protein has two short cytoplasmic domains that are required for CD36 signaling after ligand binding. (b) Ubiquitination sites (in red) in the C-terminus and palmitoylation sites (in blue) in both the C- and N-tails are highlighted. The ubiquitination sites, which are sensitive to FA and insulin, may regulate CD36 sorting to the plasma membrane and CD36 turnover. Palmitoylation might help recruit CD36 to lipid rafts and could influence interaction with Src kinases. Regulation of CD36 palmitoylation is still unknown.