Abstract
Anterior cruciate ligament (ACL) injuries are an important clinical problem, particularly for adolescent patients. The effect of skeletal maturity on the potential for ACL healing is as yet unknown. In this study, we hypothesized that fibroblastic cells from the ACLs of skeletally immature animals would proliferate and migrate more quickly than cells from adolescent and adult animals. ACL tissue from skeletally immature, adolescent, and adult pigs and sheep were obtained and cells obtained using explant culture. Cell proliferation within a collagen–platelet scaffold was measured at days 2, 7, and 14 of culture using AMMTT assay. Cellular migration was measured at 4 and 24 h using a modified Boyden chamber assay, and cell outgrowth from the explants also measured at 1 week. ACL cells from skeletally immature animals had higher proliferation between 7 and 14 days (p < 0.01 for all comparisons) and higher migration potential at all time points in both species (p < 0.01 for all comparisons).ACL cells from skeletally immature animals have greater cellular proliferation and migration potential than cells from adolescent or adult animals. These experiments suggest that skeletal maturity may influence the biologic repair capacity of intrinsic ACL cells.
Keywords: anterior cruciate ligament, skeletal maturity, adolescent, immature, fibroblast, migration
Rupture of the anterior cruciate ligament (ACL) is increasingly common among children and adolescents. 1,2 This injury is devastating, not only due to the immediate trauma, but also because an ACL-deficient knee has a significantly increased risk of premature osteoarthritis for both adolescents and young adults,3 with 78% of patients having radiographic signs of knee arthritis at only 14 years after injury, even with our current best treatment, ACL reconstruction.4 Although little is known about the long-term effect of ACL injury in children, the short-term results of ACL reconstruction in children and adolescents have been found to be inferior to that of adults,5 and thus one might suspect that long-term outcomes would be compromised as well. Thus, new solutions for this injury are actively being sought.
One promising answer for ACL injury is enhanced suture repair. In the past, “unenhanced” suture repair has been found to fail in as many as 40 to 100% of patients,6–8 and thus, enthusiasm for this procedure waned. However, studies in the last few years have demonstrated that one of the mechanisms behind the failure of the ACL to heal with suture repair may be the lack of provisional scaffolding in the wound site.9–11 With the surgical placement of a substitute provisional scaffold (a collagen–platelet composite) in the wound site at the time of suture repair, markedly improved healing of the ligament can be stimulated.12 This finding is leading to renewed enthusiasm for in vivo studies of suture repair, now enhanced with implanted biologically active scaffolds.
Characteristics of patients who may be good candidates for stimulating healing of the ligament have not yet been defined. Conventional orthopedic wisdom states that “children heal faster than adults” for fractures, and basic science studies in animals appear to support this clinical wisdom.13 As patients with open physes are vulnerable to ACL injury, and stand to have the longest period of disability if premature osteoarthritis occurs, it is clinically important to begin to examine the effect of skeletal maturity on the ability to stimulate functional healing in the ACL. In this work, we propose to start this investigation by examining specific differences between three groups of animals: skeletally IMMATURE, ADOLESCENT (approaching, but not yet skeletally mature,) and ADULT animals (closed physes)
A collagen–platelet composite scaffold (CPC) was used in this study as prior work has demonstrated that the use of a CPC scaffold can stimulate healing of both a partial and complete transection of the ACL.12,14,15 Additional studies have demonstrated that the use of platelets16 or collagen alone14 do not have the same effect; therefore, the composite was selected for use in this study.
Our first hypothesis is that fibroblastic cells from the ACL of skeletally immature animals will proliferate faster in a 3D provisional scaffold substitute than cells from the ACL of adolescent or adult animals. Our second hypothesis is that the fibroblastic cells from the ACL of skeletally immature animals will have better migration potential than cells from the ACL of adolescent or adult animals. To test this hypothesis, ACL cells from all three age groups in two large animal models will be obtained and the rate at which cells grow out from explants of tissue for each age group, as well as the rate of migration through a substrate in a modified Boyden chamber assay will be measured. If either of our hypotheses is proved, this will provide support for further study of age-related cellular differences as a possible mechanism for the age dependence of ligament healing and provide insight into possible treatment methods for improving healing in all age groups.
MATERIALS AND METHODS
Experimental Design
Skeletally IMMATURE, ADOLESCENT, and ADULT pigs and sheep were used in this study. Porcine tissue was obtained from animals used for IACUC-approved studies, and ovine tissue was obtained from other IACUC-approved studies at Children’s Hospital, Boston, with the exception of the ADOLESCENT sheep tissue, which was purchased (Research 87, Boylston, MA, USA). ACL tissue was obtained from each of the three age groups for both species and placed into explant culture. Four animals (two porcine and two ovine) were used for each age group at a designated time point, yielding a sample size of eight knees per age group and time point (sample size verification in Statistics section). An average sample size of 12 (porcine) and 5 (ovine) ACLs for explant culture was chosen based on the minimum number of explants required to obtain sufficient cell numbers to run all the assays. Primary outgrowth cells were grown to confluence and passaged. Second-passage cells were used for all assays. Proliferation within a provisional scaffold substitute was measured by seeding identical numbers of ACL cells from each age group into CPCs. Cell number within the gels was measured at days 2, 7, and 14 of culture using an MTT assay. Cellular migration was measured for all age groups using a modified Boyden chamber assay. Migration was measured at 4 and 24 h. Scaffold contraction was also observed at days 0, 2, 7, and 14 by capturing images of the gels using a Canon, Rebel XT, EOS digital camera.
Tissue Procurement
Animals were selected after consultation with a veterinary surgeon and determination that in the porcine model, physes close at approximately 14 to 18 months in the Yucatan minipig. Although sexual maturity of the Yucatans has been previously reported to be at 7 to 10 months in the Yucatan,17 it is common in animal models that skeletal maturity lags behind sexual maturity, and therefore the status of the physes were verified radiographically in all animals prior to tissue procurement and the immature animals were found to have open physes, the adolescent animals had closed tibial and femoral physes but an unfused tibial tubercle, and the ADULT animals had a physeal scar but no open physis. Therefore, in the porcine model, the ages selected (mean ± SD) were: IMMATURE (8 ± 2.1 months), ADOLESCENT (16 ± 2.2 months), and ADULT (26.1 ± 1.1 months).
The sheep model used in these studies was a Dorset Cross. In this model, the physes of the ewes close between 9 and 12 months (USDA Agricultural Marketing Service Report, 1982). Therefore, the ages selected (mean ± SD) in this model were: IMMATURE (7 ± 1 day), ADOLESCENT (6.5 ± .5 months), and ADULT (15 ± 0.125 months).
The ACLs for porcine and ovine animals were harvested from the knees using sterile technique. The animals were premedicated with telazol, xylazine, and atropine followed by intubation. Anesthesia was maintained with 1–3% isoflurane. Both limbs were shaved, prepared with Betadyne, and sterilely draped. An incision 4 cm in length was made at the medial border of the patellar tendon using a No.15 blade. The medial retinaculum was divided at the patellar tendon border. The patella was gently retracted laterally and the fat pad was resected to expose the ACL. Biopsies of ACL tissue were taken from the middle third of the ligament for all of the retrievals. Care was taken to avoid the insertion sites. The ACL tissue was placed into phosphate-buffered saline (PBS) and brought to the sterile hood where it was cut into explants. The number of ligaments harvested and the number of explants obtained from each group of animals are listed in Table 1.
Table 1.
Total Number of Porcine and Ovine ACLs Acquired After Surgery and the Number of Total Explants Used to Obtain Enough Second Passage Cells to Complete the Migration and Proliferation Assays
| Group | Number of ACLs |
Number of Explants |
|---|---|---|
| Pig—IMMATURE | 12 | 288 |
| Pig—ADOLESCENT | 12 | 288 |
| Pig—ADULT | 12 | 288 |
| Sheep—IMMATURE | 6 | 72 |
| Sheep—ADOLESCENT | 4 | 192 |
| Sheep—ADULT | 4 | 96 |
Cell Culture
After harvest, explants were washed three times in 10% Antibiotic–Antimycotic solution (AB/AM) (Mediatech Inc., Cat. #30-004-Cl, Herndon, VA, USA) followed by three washes with sterile 1× PBS (EMD Chemicals, Cat. #: B10241-34, Gibbstown, NJ, USA) and transferred onto 35-mm well plates with six wells per plate. The explants were allowed to adhere to the plates and then media was slowly added. Explants were cultured in complete media containing Dulbecco’s Modification of Eagle’s Medium (DMEM) (Mediatech, Inc., Cat. #10-013-CV) 10% fetal bovine serum (FBS) (HyClone Inc., Cat. # 16777-006, South Logan, UT, USA) and 1% AB/AM. The explants were maintained in culture, and the media was changed two times per week.
When the primary outgrowth cells were 80% confluent, they were trypsinized and frozen until all age groups had been collected for the experiment. Cells were frozen at 1 million cells per milliliter media solution in cryogenic vials and stored at −80°C until use. After defrosting, the cell solutions were seeded sterilely into T-75 flasks with a seeding density of 2.1 × 106 cells per flask. Complete media was added to complete the volume to 12 mL. The cells were maintained in culture with medium changes two times per week until 80% confluence was achieved. Cells were passaged a second time before use and all cells used for this experiment were second passage.
Preparation of Collagen-PRP Cell-Seeded Provisional Scaffold
Acid-soluble, Type I collagen slurry was made by sterilely harvesting bovine knee capsular tissue that was solubilized in an acidic solution as previously described.14 Collagen content within the slurry was adjusted to greater than 5 mg/mL and neutralized with 0.1M HEPES (Cellgro, Mediatech, Inc, Herndon, VA), 5× PBS (HyClone), and enough 7.5% sodium bicarbonate (Cambrex BioScience Walkersville, Inc., Walkersville, MD, USA) to obtain a final pH of 7.4.
Platelet-rich plasma (PRP) was prepared for each species. Porcine platelets were prepared from 60 mL of whole blood drawn from one hematologically normal Yucatan pig under general anesthesia. Blood was collected in a bag with 10% by volume acid–citrate dextrose from Animal Resources at Children’s Hospital (ARCH) in Boston, MA. The blood was centrifuged for 6 min at 150 × g (GH 3.8 rotor, Beckman GS-6 Centrifuge, Fullerton, CA, USA). The supernatant was aspirated and collected as PRP in a 50-mL tube. Complete blood counts (CBCs) were performed on the whole blood and the platelet solution. Ovine platelet solution was prepared using 60 mL of whole blood drawn from one hematologically normal sheep under general anesthesia. Blood was collected in a bag with 10% by volume acid–citrate dextrose from ARCH in Boston, MA. A Harvest Technologies SmartPReP2 centrifugation system (Harvest Technologies, Plymouth, MA, USA) with a 20-min spin time was used to prepare the platelet solution. CBCs were performed on all whole blood and platelet solution. Second passage ACL cells from each age group were trypsinized and resuspended in platelet solution at a density of 1.5 × 106 cells/mL.
The CPCs were made by combining the cell-seeded platelet solution and cold, neutralized collagen solution. Each 0.5-mL CPC contained 0.19 mL cell-seeded PRP and 0.31 mL collagen-buffer solution and 7.5 × 105 cells per gel. The collagen solution was transferred into silicone semitubular molds with polyethylene mesh at each end to anchor the gels within six well plates using a repeat pipettor. This was done to ensure uniform volume across all scaffolds. The scaffolds were incubated at 37°C for 1 h, to ensure complete gelation. After gelation, 6mL of complete media was added to each well to cover the composites. Samples were cultured in a 37°C CO2 humidified incubator and the complete media was changed twice per week.
MTT Assay
Fibroblast number in the CPCs was determined at day 2, 7, and day 14 by using the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay; an assay that measures the ability of a cell’s mitochondrial dehydrogenase enzymes to convert yellow soluble MTT salt into purple formazan salt. MTT powder (USB Corporation, Cleveland, OH, USA) was mixed with PBS at a ratio of 1 mg/mL for this assay. A total of 81 CPCs were made for each species, with eight cell-seeded scaffolds analyzed at each time point (2, 7, and 14 days) for each age group (IMMATURE, ADOLESCENT, and ADULT) and each species (porcine and ovine). In addition, three cell-free control scaffolds were analyzed at each time point for each species. The mean and standard deviation in prior pilot experiments (unpublished) for the cell free controls (0.054 ± 0.24) was very small (as found here) and thus, fewer of these controls were needed to have adequate power to detect significant differences between groups.
At the designated time point, the media was aspirated from each well, and the scaffolds were removed from the silicone semitubular molds and placed into individual wells of 12 well plates. One milliliter of the 1 mg/mL MTT solution was added to each well. Each gel was fully immersed in the MTT solution. After the MTT was added, the plates were incubated for 3 h at 37°C and 5% CO2. Subsequently, the excess MTT solution was removed and 1mLof sterile 1× PBS was added to each well. The plates were then placed on a horizontal agitator (Fisher Scientific Clinical Rotator, 100 rpm) and left to rinse at room temperature for 30 min. Rinses were repeated until absorbencies of the wash were less than 0.100. All PBS was then removed and each gel transferred with a sterile spatula into a sterile 5.0-mL centrifuge tube. The gels were then placed in 1 mL of a detergent containing 20% aqueous SDS/formamide (1:1 volume ratio) in each tube and incubated overnight in at 37°C. Finally, the tubes were vortexed on high for 5 s and then centrifuged for 5 min at 1500 rpm. Aliquots (200 µL) of the supernatant from each tube were then transferred onto a sterile 96-well plate. The absorbencies were measured at 562 and 650 nm, and the relative numbers of fibroblasts were determined. We did not measure apoptosis along with proliferation, and therefore, our MTT cell number results reflect the net effect of proliferation and cell loss.
Provisional Scaffold Size
Images of the gels were captured at days 0, 2, 7, and 14 using a Canon, Rebel XT, EOS digital camera. The area of each scaffold was measured at each day using NIH Image J 1.37V. For each group, the scaffold areas were averaged and the mean and standard deviation reported. In addition, the percent of day 0 size was calculated at each subsequent day.
Cellular Migration
Outgrowth Measures
After harvest, explants were washed in 10% antibiotic-antimycotic solution (AB/AM) (Mediatech Inc., Cat. #30-004-Cl) and sterile 1× PBS (EMD Chemicals, Cat. #: B10241-34, Gibbstown, NJ, USA). The ligament was measured and one strip was cut from the proximal half for histology, and the remaining femoral half of the ligament was cut into approximately 50 explants, each measuring to be 2 × 2 × 2 mm. The explants were allowed to adhere to the plates for 10–15 min, or until they were firmly in place. Explants were cultured in complete media containing DMEM (Mediatech, Inc., Cat. #10-013-CV) 10% FBS (HyClone Inc., Cat. # 16777-006) and 1% AB/AM. Approximately 1 mL, or enough to just cover the explants, was added to each well. The explants were maintained in culture, and the media was changed two times per week. Outgrowth was measured using a prepared checkered transparency grid and an inverted microscope (4× objective) once per week for 3 weeks. Cells for each age group were counted until confluency.
Modified Boyden Chamber Assay
Cell migration was measured using the modified Boyden chamber assay (Chemicon International Inc. Kit: QCM 24-well colorimetric cell migration assay). The upper and lower chambers are separated by an 8 µm polycarbonate membrane and the migration through this membrane from the upper to lower chamber is measured using a cell stain solution. For each time point (4 and 24 h) and each age group (IMMATURE, ADOLESCENT, and ADULT) a sample size of n = 8 were analyzed in triplicate.
Serum-free quenching medium was added to each T-75 flask (Serum-free DMEM containing 10% FBS) and incubated for 24 h prior to assay. After 24 h, cells were washed with sterile PBS (EMD Chemicals, Cat. #: B10241-34). Five milliliters of harvesting buffer [0.05% trypsin in Hanks Balanced Salt Solution (HBSS) containing 25 mM HEPES, Cellgro, Mediatech, Inc.] was added to the cells and incubated at 37°C for 15 min. After 15 min, cells were gently pipetted off the dish and 15 mL of quenching medium (Serum-free DMEM containing 10% FBS) was added. Cells were centrifuged for 5 min at 1200 rpm until pellets were formed. Pellets were resuspended in 5mL of quenching medium [serum-free DMEM containing 5% bovine serum albumin (BSA)]. Three hundred microliters of cell suspension [0.5–1.0 × 106 cells/mL in chemoattractant-free media (serum-free DMEM containing 5% BSA) and 500 µL of serum free media (serum-free DMEM)] were added to each insert. Inserts were handled with forceps sterilized with 70% ethanol. Plates were covered and incubated for 4 to 24 h (37°, 5% CO2). Prior pilot studies in our laboratory demonstrated that ACL cell migration can be detected by this assay as early as 4 h after initiation, and that little change was noted in the amount of measurable migration after 24 h, most likely because the filter would reach saturation shortly after that time point. Therefore, a short (4 h) and longer (24 h) time point were both selected in this study. At the designated time points, cells were removed from the top side of the insert by pipetting out the remaining cell suspension and placing the inset into a clean well containing 400 µL of cell stain (part no. 90144). Inserts were air dried for 20 min at room temperature. Inserts were dipped into a clean well with water to rinse. Promptly after rinsing, the cells were removed from the interior of the insert using cotton-tipped swabs. Inserts were air dried for 20 min and then transferred into a clean well containing 200 µL of extraction buffer (part no. 90145) for 15 min at room temperature. Stain was extracted by tilting insert back and forth several times during incubation. Inserts were removed from the well and the mixture was transferred onto a 96-well microtiter plate from which the optical density was read at 562 nm.
Statistics
Statistical methods for analysis of the data include a two-way analysis of variance (ANOVA), linear regression and construction of 95% confidence intervals using a normal approximation with use of SPSS statistics package (Version 13.0, SPSS Inc., Chicago, IL, USA) All reported p values are two tailed.
We based our sample size calculation on a minimal effect size of 2, equaling a difference in proliferation, migration, or contraction of 20% between age groups with a standard deviation of 10%, as was seen in pilot studies, and accounted for three subgroups. Under these parameters, a sample size of n = 6 would result in a power of 80%, n = 7 in 85%, and n = 8 in a power larger than 90%, whereas the addition of further samples increased the power only marginally per animal. All these calculations were performed using Intercooled STATA 10 (Statacorp LP, College Station, TX, USA). Thus, we chose a sample size of 8 to combine maximal power with minimal animal sacrifice. Three cell-free control scaffolds were examined at each age.
RESULTS
Cellular Proliferation
Platelet Solution Preparation
The platelet cell count (Plt), white blood cell count (WBC), and the red blood cell count (RBC) in the whole blood and platelet solution are reported as follows in Table 2. The enrichment factor is the ratio between the platelet concentration in the PRP and that in the systemic whole blood of the animal. The enrichment factor in the porcine experiments was 3.1×, whereas in the sheep experiments it was 2.8×.
Table 2.
Platelet, White Cell, and Red Cell Counts in the Whole Blood and Platelet-Rich plasma Samples
| Blood Fraction | Porcine Whole Blood | Porcine PRP | Ovine Whole Blood | Ovine PRP |
|---|---|---|---|---|
| Plt (109/L) | 293 | 915 | 131 | 369 |
| WBC (109/L) | 17.44 | 5.37 | 59.7 | 9.6 |
| RBC (1012/L) | 5.91 | 0.07 | 6.37 | 0.14 |
MTT Assay
In the porcine model, at day 14, fibroblast number was significantly greater in IMMATURE porcine cell-seeded scaffolds when compared to ADULT porcine cell-seeded scaffolds (Fig. 1A, p = 0.010), and ADOLESCENT porcine cell-seeded scaffolds (p = 0.044). There was no significant difference between ADOLESCENT and ADULT at day 14 (p > 0.99). Fibroblast cell number increased within the gels between day 2 and day 14 for all age groups and both species (p < 0.0001 for all comparisons, cell free controls reported as 0.077 ± 0.37).
Figure 1.
(A) Cell number of IMMATURE, ADOLESCENT, and ADULT porcine composites at days 2, 7, and 14. Mean absorbance in the MTT assay correlated directly with cell number. Values are expressed as mean ± SD, with n = 8. * Indicates a significant difference between IMMATURE and ADOLESCENT cell (p = 0.010), # indicates a significant difference between IMMATURE and ADULT cells (p = 0.044). (B) Cell number of IMMATURE, ADOLESCENT, and ADULT ovine composites at days 2, 7, and 14. Mean absorbance in the MTT assay correlated directly with cell number. Values are expressed as mean ± SD, with n = 8. *Indicates a significant difference between IMMATURE and ADOLESCENT cells (p = 0.0091), # indicates a significant difference between IMMATURE and ADULT cells (p < 0.0001), ♠ indicates a significant difference between ADOLESCENT and ADULT cells (p = 0.007).
Similar results were found in the ovine model where at day 14, the fibroblast number in the IMMATURE ovine cell-seeded scaffolds was significantly greater than the fibroblast number for both the ADOLESCENT and ADULT ovine cell-seeded scaffolds (Fig. 1B, p = 0.0091 and p < 0.0001, respectively). Also, there was a significantly greater number of fibroblasts in ADOLESCENT ovine cell-seeded scaffolds than ADULT ovine cell-seeded scaffolds (p = 0.007). Fibroblast number in the CPCs was not significantly different among any of the age groups at day 2 for either the porcine or ovine cells. There was also no difference at day 7 (p > 0.297 for all comparisons).
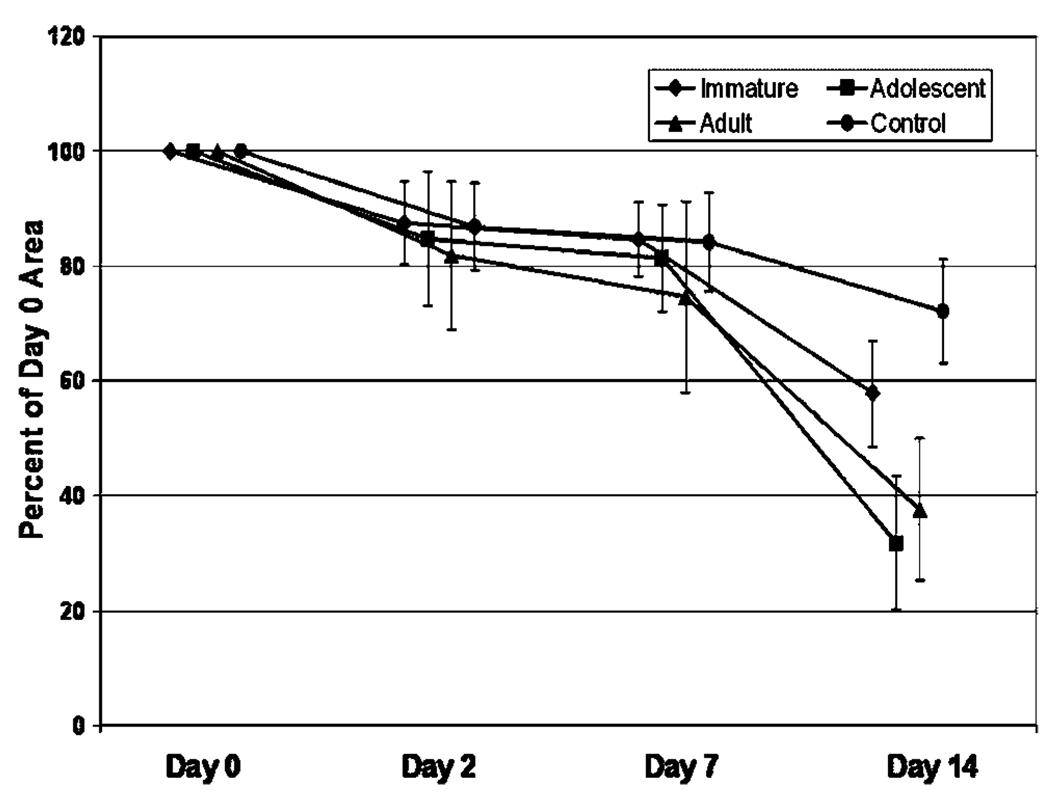
Scaffold Contraction
At 2 days after cell seeding, the CPCs in all seeded and control groups were the same size (Fig. 2, p > 0.08 for all comparisons). For the porcine cells, there were only minimal differences seen in the contraction rates for the three age groups over the 2-week culture period. However, in the ovine groups, at 14 days, the CPCs seeded with ADOLESCENT and ADULT cells were 48 and 37% smaller than those seeded with IMMATURE cells, differences that were both statistically significant (p < 0.0001 for both comparisons). All three groups were smaller than the unseeded control CPCs (p < 0.002 for all comparisons). The majority of the contraction in the three groups occurred between 7 and 14 days (Fig. 2).
Figure 2.
Ovine cell provisional scaffold contraction. Percent of day 0 area is represented at days 0, 2, 7, and 14. Note the increased rate of contraction in the ADOLESCENT and ADULT scaffolds when compared with the scaffolds seeded with IMMATURE ACL cells (p < 0.0001 for both comparisons at day 14). Error bars represent one standard deviation.
Cellular Migration
Porcine Cell Outgrowth Study
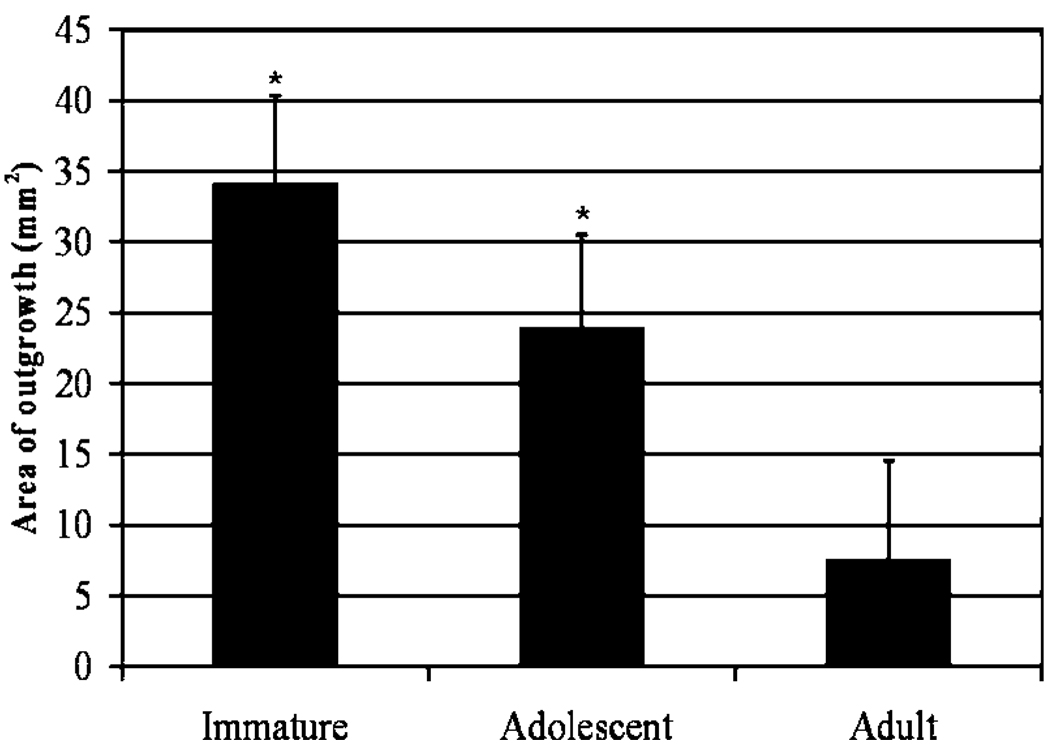
IMMATURE cells had the largest area of outgrowth at week 1, followed by ADOLESCENT and then ADULT cells (Fig. 3). The area of outgrowth of the ACL cells was greatest for IMMATURE cells at weeks 1 and 2; followed by ADOLESCENT and ADULT. At week 1, ADULT cells covered 7.5 ± 7 mm2 of the culture surface area, which was significantly lower than the area covered by the IMMATURE (34 ± 6.32 mm2, p < 0.0001) and ADOLESCENT cells (23.83 ± 6.68 mm2, p < 0.001). By week 2, the culture surface area for all age groups was near confluency, and by week 3, all age groups were completely confluent.
Figure 3.
Area covered by cells growing from ACL explants cultured in complete medium at week 1. The values represent the square area of the culture dish covered by the outgrowing layer of cells from each individual ACL explant. Values are expressed as area of outgrowth ± SD, where * indicates IMMATURE and ADOLESCENT cell coverage was significantly different from ADULT outgrowth, p < 0.001 and p < 0.05.
Boyden Chamber Assay Study
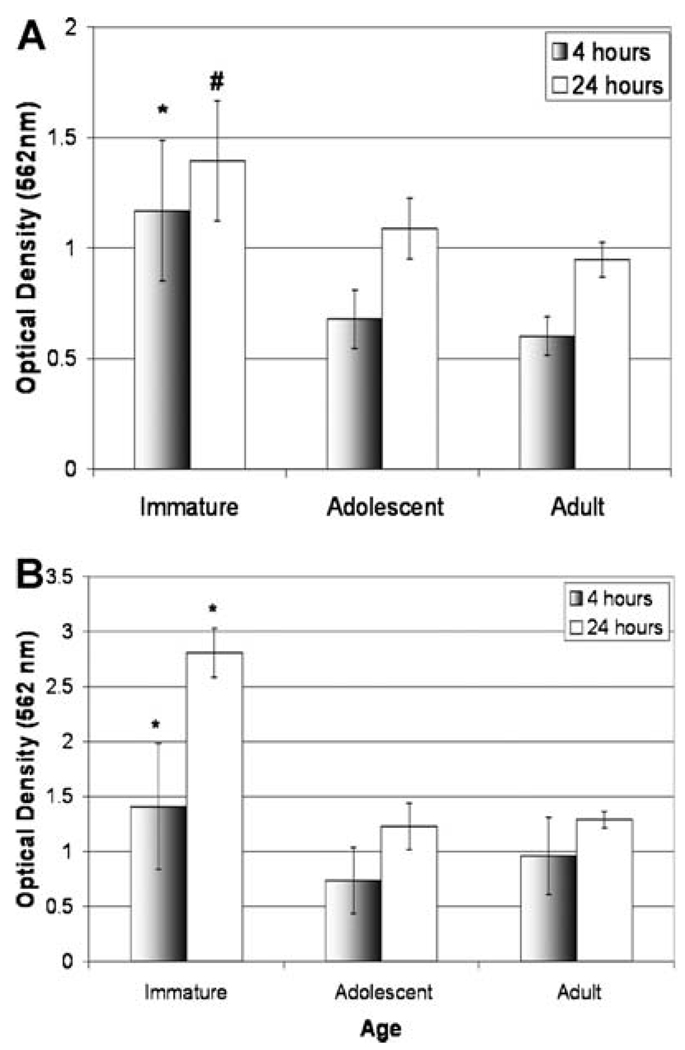
Porcine ACL cells: cells obtained from IMMATURE porcine ACLs had a significantly higher rate of migration than both ADOLESCENT and ADULT cells (Fig. 4A). The IMMATURE cells had a 170% significantly higher migration rate than the ADOLESCENT cells at both 4 and 24 h (p < 0.0001 and p = 0.008, respectively) and a 190% higher rate of migration than the ADULT cells at both 4 and 24 h (p < 0.0001 for both time points, respectively). There was no statistically significant differences among ADOLESCENT and ADULT at either time point (p > 0.99 and p = 0.452, respectively).
Figure 4.
(A) Cell migration of IMMATURE, ADOLESCENT, and ADULT porcine cells at 4 and 24 h. Results reported as optical density, which correlates directly with cell number using this assay. Values are expressed as mean ± SD, where n = 8. * Indicates IMMATURE cells migrated significantly more than ADOLESCENT and ADULT cells at 4 h, p < 0.001. # indicates IMMATURE cells migrated significantly more than ADOLESCENT (p < 0.01) and ADULT cells (p < 0.001) at 24 h. (B) Cell migration of IMMATURE, ADOLESCENT, and ADULT sheep cells at 4 h. Results reported as optical density, which correlates directly with cell number using this assay. Values are expressed as mean ± SD, where n = 8. * Indicates IMMATURE cells migrated significantly faster than cells from ADOLESCENT and ADULT ACLs at 4 and 24 h, p < 0.01.
Ovine ACL cells: cells obtained from IMMATURE ovine ACLs had a significantly higher rate of migration at 4 and 24 h when compared with ADOLESCENT and ADULT cells (Fig. 4B, p < 0.01). There were no statistically significant differences among ADOLESCENT and ADULT at 4 or 24 h (p = 0.653 and p = 0.207).
DISCUSSION
The results of this in vitro study suggest that in two large animal models, porcine and ovine, cells taken from skeletally IMMATURE animals have greater proliferation and migration potential than ADOLESCENT and ADULT cells. These results support previous reports that the migratory and proliferative ability of fibroblasts is age dependent.18–23 Both the proliferation and migration of intrinsic ACL cells has been cited as a prerequisite for proper wound healing20,24 and thus our findings might be a reason why IMMATURE animals could be able to heal more quickly and efficiently than older age groups.
Invasion of the wound site by fibroblasts is critical to the formation of fibrovascular scar.25 As an early response to injury, fibroblasts will migrate toward the wound site.26 Previous studies have shown that defective wound healing occurs when migration potential is less than optimal.27,28 The decrease in migration rates seen in adolescence and adulthood could thus potentially also represent a mechanism for decreased healing potential of the ACL after minor partial injuries that may occur as a normal part of daily “wear and tear.” If such a deficiency in repair were present, it could lead to an imbalance in the normal tissue remodeling that occurs in all tissues, an imbalance toward continual breakdown with insufficient repair and gradual weakening of the tissue until failure.
Platelets were used in this study in an attempt to try to simulate the clinical situation where a fibrin platelet clot forms in a wound site as the first step in wound healing. Prior in vitro experiments have demonstrated that the CPC releases growth factors important in wound healing over a 10-day period.29,30 Thus, it is possible that the platelets used in this in vitro experiment also released these growth factors and subsequently produced a growth factor environment more similar to that seen in the in vivo healing wound than constructs without platelets or cultured with static concentrations of growth factors in PBS. Certainly, the in vitro nature of our study limits is direct application to clinical use, and this is the primary limitation of our study. Another limitation is that we did not study older adult animals. The ages of animals used are more likely comparable to younger age groups of humans—the widely open physes of the IMMATURE animals would correspond to a human patient less than 10 years of age, the closing physes of the ADOLESCENT animals would be similar to that seen in humans between 12 and 16 years of age, and the relatively recently closed physes in the ADULT group would correspond to young adults in their third and fourth decade of life.
In addition to migration, proliferation and contraction of cells at the wound site characterizes a major component of wound healing.24,26 Increased cellular proliferation is required early in the repair process of the ACL for appropriate stages of wound healing to take place. Fibroblast proliferation is often accompanied by matrix synthesis, which together mark the onset of the proliferation phase. Histological analysis has shown that the fibroblast becomes the most dominant cell type 3 weeks postinjury, allowing for the wound to heal, and is then followed by a rapid decline at 6 weeks, marking the remodeling phase.31–33 The values of the 2-day MTT assay were similar in all three age groups for both species. This suggests that the initial seeding density was similar for each age group in the proliferation experiments. The higher values seen in the immature animals at later time points thus suggests a higher rate of cell proliferation by cells from the younger animals. These results are consistent with prior studies of fibroblast proliferation where fibroblasts from older donors show a decrease in replication rates and lower cell yields at cellular confluency in vitro.34 Taken in total, these results suggest that an increase in age results in lower rates of cell migration, proliferation, and contraction, and potentially negatively impact the capacity of a wound to heal after injury.
Interestingly, the same effect of age was seen in both species, where skeletally immature animals had the highest rates of migration and proliferation, and adult animals had the lowest. The similarity in their skeletal maturity status and the similarity in the findings in both species do make it more likely that these findings are applicable across species rather than species-specific. If these findings are applicable across species, and perhaps even applicable in the human situation, it might suggest that one reason for the relatively accelerated healing noted in children after injury when compared with adults is the enhanced ability of cells from immature tissues to migrate and proliferate to and within the wound site. Clearly, further studies are needed to validate this hypothesis. In addition, the age-related differences in cell proliferation and scaffold contraction were significant at day 14. Whether these differences persist at longer time points is as yet unknown. Hydrogel studies of this type are often limited in the length of time that can be studied, as reorganization and contraction of the hydrogels can lead to premature failure of the scaffolds and invalidate the study. Thus, for studying these effects at longer time points, future studies using an in vivo model of ACL repair may be needed.
Although the differences between age groups for the two species were consistent, there were observed differences in the magnitude of the measured cellular proliferation between porcine and ovine assays. These differences could be due to at least two different factors: first, that sheep cells migrate much faster than the porcine cells or second, that each sheep cell that migrates takes up a greater amount of dye than each porcine cell. We do not know if it is one of these factors, or another unidentified factor which is responsible for this observation. However, as the principal goal of our study was to determine not the difference between species, but the differences as a function of skeletal age, it was gratifying to note that the trends in cellular proliferation and migration observed as a function of age were similar in both species, suggesting that the age dependence may not be true only in one animal model, but across species.
It is interesting to note that although patients who are skeletally immature and prepubescent are certainly active, the incidence of ACL tears in these athletes is far lower than that in adolescents or adults.35 Perhaps some of these differences in catastrophic injury rates can be attributed to intrinsic factors, such as faster migration of cells to wound sites, which may allow these equally active patients to more effectively repair microstrain or individual fascicle disruption which may occur semi-regularly, thus avoiding accumulation of these small defects and eventual coalescence into a complete disruption.
One limitation to this study is the use of animal models. As it is very difficult to obtain healthy ACL cells from a patient with no knee injury, we worked to mitigate this limitation by selecting two animal models that are thought to most closely approach the human condition. The age groups chosen for the porcine and ovine models used in this study are anatomically similar in their epiphyseal (growth) plate growth. The time points chosen correlate for both species so that the immature animals have an open growth plate, adolescent is partially open and adults have a closed growth plate. The porcine model has the most similar healing characteristics to human in skin. It is commonly used for wound healing studies36 and also has similar anatomy and biomechanics to the human knee,37 whereas sheep are one of the most common models for ACL reconstruction, 38,39 and the placement of its anteromedial and posterolateral bundles are similar to that of a human model.40 The fibroblasts cultured from these two animals were passaged twice prior to the experiment. We chose a low passage because it has been shown that late passaged fibroblasts display an increased nuclear size, which correlates with slow and nondividing cells and thus, higher passage cells may be a less accurate representation of the in vivo condition.34,41 We used two large animal models, as we felt that if similar results were seen in both models that the results were less likely to be species-dependent. If different results were noted, we might have tested the results in a third species or looked for other physiologic reasons the responses were different (different weight gain curves, different timing of sexual maturity in relationship to skeletal maturity, etc). The similarity in their skeletal maturity status and the similarity in the findings in both species do make it more likely that these findings are applicable across species rather than species-specific, but further studies would be required to validate that hypothesis.
The chief limitation of this study is its in vitro nature. The environment of the culture dish is very different from that of the in vivo environment, and although the in vitro model is useful to begin to understand intrinsic ACL cell migration behaviors, there are likely many other cell types involved in governing the wound response, including inflammatory cells found in the blood. These cells are excluded from this in vitro assay, and therefore future studies should include in vivo studies where the effects of the intrinsic ACL cells are more difficult to isolate and ascertain, but the cumulative invasion characteristics of all the cells in the in vivo wound environment can be determined as a function of animal age. Although additional studies are needed, these experiments suggest the role of skeletal maturity may influence the repair capacity of intrinsic ACL cells.
ACKNOWLEDGMENTS
This study was supported by a grant from the National Institutes of Health (NIAMS R01 AR054099 and K02 AR049346 to M.M.M.). Dr. Murray serves on the scientific advisory board and as a consultant for Connective Orthopaedics, and also owns stock in that same company.
REFERENCES
- 1.McIntosh AL, Dahm DL, Stuart MJ. Anterior cruciate ligament reconstruction in the skeletally immature patient. Arthroscopy. 2006;22:1325–1330. doi: 10.1016/j.arthro.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 2.Johnston DR, Baker A, Rose C, et al. Long-term outcome of MacIntosh reconstruction of chronic anterior cruciate ligament insufficiency using fascia lata. J Orthop Sci. 2003;8:789–795. doi: 10.1007/s00776-003-0708-9. [DOI] [PubMed] [Google Scholar]
- 3.Lohmander LS, Ostenberg A, Englund M, et al. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. 2004;50:3145–3152. doi: 10.1002/art.20589. [DOI] [PubMed] [Google Scholar]
- 4.Von Porat A, Roos EM, Roos H. High prevalence of osteoarthritis 14 years after an anterior cruciate ligament tear in male soccer players: a study of radiographic and patient relevant outcomes. Br J Sports Med. 2004;38:263. doi: 10.1136/ard.2003.008136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aichroth PM, Patel DV, Zorrilla P. The natural history and treatment of rupture of the anterior cruciate ligament in children and adolescents. A prospective review. J Bone Joint Surg Br. 2002;84:38–341. doi: 10.1302/0301-620x.84b1.11773. [DOI] [PubMed] [Google Scholar]
- 6.Feagin JA, Jr, Curl WW. Isolated tear of the anterior cruciate ligament: 5-year follow-up study. Am J Sports Med. 1976;4:95–100. doi: 10.1177/036354657600400301. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan N, Wickiewicz TL, Warren RF. Primary surgical treatment of anterior cruciate ligament ruptures: a long-term follow-up study. Am J Sports Med. 1990;18:254–358. doi: 10.1177/036354659001800404. [DOI] [PubMed] [Google Scholar]
- 8.Sherman MF, Bonamo JR. Primary repair of the anterior cruciate ligament. Clin Sports Med. 1988;7:739–750. [PubMed] [Google Scholar]
- 9.Murray MM, Martin SD, Martin TL, et al. Histological changes in the human anterior cruciate ligament after rupture. J Bone Joint Surg Am. 2000;82-A:1387–1397. doi: 10.2106/00004623-200010000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Murray MM, Spector M. The migration of cells from the ruptured human anterior cruciate ligament into collagen-glycosaminoglycan regeneration templates in vitro. Biomaterials. 2001;22:2393–2402. doi: 10.1016/s0142-9612(00)00426-9. [DOI] [PubMed] [Google Scholar]
- 11.Spindler KP, Murray MM, Devin C, et al. The central ACL defect as a model for failure of intra-articular healing. J Orthop Res. 2006;24:401–406. doi: 10.1002/jor.20074. [DOI] [PubMed] [Google Scholar]
- 12.Murray MM, Spindler KP, Ballard P, et al. Enhanced histologic repair in a central wound in the anterior cruciate ligament with a collagen-platelet-rich plasma scaffold. J Orthop Res. 2007;25:1007–1017. doi: 10.1002/jor.20367. [DOI] [PubMed] [Google Scholar]
- 13.Cullen MC, Roy DR, Crawford AH, et al. Open fracture of the tibia in children. J Bone Joint Surg Am. 1996;78:1039–1047. doi: 10.2106/00004623-199607000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Murray MM, Spindler KP, Abreu E, et al. Collagen-platelet rich plasma hydrogel enhances primary repair of the porcine anterior cruciate ligament. J Orthop Res. 2007;25:81–91. doi: 10.1002/jor.20282. [DOI] [PubMed] [Google Scholar]
- 15.Murray MM, Spindler KP, Devin C, et al. Use of a collagen-platelet rich plasma scaffold to stimulate healing of a central defect in the canine ACL. J Orthop Res. 2006;24:820–830. doi: 10.1002/jor.20073. [DOI] [PubMed] [Google Scholar]
- 16.Murray MM, Palmer M, Abreu E, et al. Platelet-rich plasma alone is not sufficient to enhance suture repair of the ACL in skeletally immature animals: an in vivo study. J Orthop Res. 2008 doi: 10.1002/jor.20796. (accepted for publication) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sebert SP, Lecannu G, Kozlowski F, et al. Childhood obesity and insulin resistance in a Yucatan mini-piglet model: putative roles of IGF-1 and muscle PPARs in adipose tissue activity and development. Int J Obes (Lond) 2005;29:324–333. doi: 10.1038/sj.ijo.0802823. [DOI] [PubMed] [Google Scholar]
- 18.Kondo H, Yonezawa Y. Changes in the migratory ability of human lung and skin fibroblasts during in vitro aging and in vivo cellular senescence. Mech Ageing Dev. 1992;63:223–233. doi: 10.1016/0047-6374(92)90001-t. [DOI] [PubMed] [Google Scholar]
- 19.Pienta KJ, Coffey DS. Characterization of the subtypes of cell motility in ageing human skin fibroblasts. Mech Ageing Dev. 1990;56:99–105. doi: 10.1016/0047-6374(90)90001-v. [DOI] [PubMed] [Google Scholar]
- 20.Mogford JE, Tawil N, Chen A. Effect of age and hypoxia on TGFbeta1 receptor expression and signal transduction in human dermal fibroblasts: impact on cell migration. J Cell Physiol. 2002;190:259–265. doi: 10.1002/jcp.10060. [DOI] [PubMed] [Google Scholar]
- 21.Norrby K, Bergstrom S, Druvefors P. Age-dependent mitogenesis in normal connective tissue cells. Virchows Arch. 1981;36:27–34. doi: 10.1007/BF02912051. [DOI] [PubMed] [Google Scholar]
- 22.Drubaix I, Giakoumakis A, Robert L, et al. Preliminary data on the age-dependent decrease in basic fibroblast growth factor and platelet-derived growth factor in the human vein wall and in their influence on cell proliferation. Gerontology. 1998;44:9–14. doi: 10.1159/000021976. [DOI] [PubMed] [Google Scholar]
- 23.Schneider EL, Mitsui Y. The relationship between in vitro cellular aging and in vivo human age. Proc Natl Acad Sci USA. 1976;73:3584–3588. doi: 10.1073/pnas.73.10.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raghow R. The role of extracellular matrix in post-inflammatory wound healing and fibrosis. FASEB J. 1994;8:823–831. doi: 10.1096/fasebj.8.11.8070631. [DOI] [PubMed] [Google Scholar]
- 25.Steinbrech DS, Longaker MT, Mehrara BJ, et al. Fibroblast response to hypoxia: the relationship between angiogenesis and matrix regulation. J Surg Res. 1999;84:127–133. doi: 10.1006/jsre.1999.5627. [DOI] [PubMed] [Google Scholar]
- 26.Martin P. Wound healing—aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 27.Denton CP, Khan K, Hoyles RK, et al. Inducible lineage-specific deletion of TbetaRII in fibroblasts defines a pivotal regulatory role during adult skin wound healing. The J Invest Dermatol. 2008 doi: 10.1038/jid.2008.171. [DOI] [PubMed] [Google Scholar]
- 28.Kurosaka S, Kashina A. Cell biology of embryonic migration. Birth Defects Res C Embryo Today. 2008;84:102–122. doi: 10.1002/bdrc.20125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fufa D, Shealy B, Jacobson M, et al. Activation of platelet-rich plasma using soluble type I collagen. J Oral Maxillofac Surg. 2008;66:684–690. doi: 10.1016/j.joms.2007.06.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacobson M, Fufa D, Abreu EL, et al. Platelets, but not erythrocytes, significantly affect cytokine release and scaffold contraction in a provisional scaffold model. Wound Repair Regen. 2008;16:370–378. doi: 10.1111/j.1524-475X.2008.00376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidt CC, Georgescu HI, Kwoh CK, et al. Effect of growth factors on the proliferation of fibroblasts from the medial collateral and anterior cruciate ligaments. J Orthop Res. 1995;13:184–190. doi: 10.1002/jor.1100130206. [DOI] [PubMed] [Google Scholar]
- 32.Frank C, Woo SL, Amiel D, et al. Medial collateral ligament healing. A multidisciplinary assessment in rabbits. Am J Sports Med. 1983;11:379–389. doi: 10.1177/036354658301100602. [DOI] [PubMed] [Google Scholar]
- 33.Frank C, Amiel D, Woo SL, et al. Normal ligament properties and ligament healing. Clin Orthop Relat Res. 1985;196:15–25. [PubMed] [Google Scholar]
- 34.Reff M, Schneider EL. Cell culture aging. Molecular and cellular biochemistry. 1981;36:169–176. doi: 10.1007/BF02357034. [DOI] [PubMed] [Google Scholar]
- 35.Kocher MS, Smith JT, Zoric BJ, et al. Transphyseal anterior cruciate ligament reconstruction in skeletally immature pubescent adolescents. J Bone Joint Surg Am. 2007;89:2632–2639. doi: 10.2106/JBJS.F.01560. [DOI] [PubMed] [Google Scholar]
- 36.Sullivan TP, Eaglstein WH, Davis SC, et al. The pig as a model for human wound healing. Wound Repair Regen. 2001;9:66–76. doi: 10.1046/j.1524-475x.2001.00066.x. [DOI] [PubMed] [Google Scholar]
- 37.Xerogeanes JW, Fox RJ, Takeda Y, et al. A functional comparison of animal anterior cruciate ligament models to the human anterior cruciate ligament. Ann Biomed Eng. 1998;26:345–352. doi: 10.1114/1.91. [DOI] [PubMed] [Google Scholar]
- 38.Nuss KM, Auer JA, Boos A, et al. An animal model in sheep for biocompatibility testing of biomaterials in cancellous bones. BMC Musculoskelet Disord. 2006;7:67. doi: 10.1186/1471-2474-7-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goradia VK, Rochat MC, Grana WA, et al. Tendon-to-bone healing of a semitendinosus tendon autograft used for ACL reconstruction in a sheep model. Am J Knee Surg. 2000;13:143–151. [PubMed] [Google Scholar]
- 40.Radford WJ, Amis AA, Kempson SA, et al. A comparative study of single- and double-bundle ACL reconstructions in sheep. Knee Surg Sports Traumatol Arthrosc. 1994;2:94–99. doi: 10.1007/BF01476480. [DOI] [PubMed] [Google Scholar]
- 41.Mitsui Y, Schneider EL. Increased nuclear sizes in senescent human diploid fibroblast cultures. Exp Cell Res. 1976;100:147–152. doi: 10.1016/0014-4827(76)90336-0. [DOI] [PubMed] [Google Scholar]