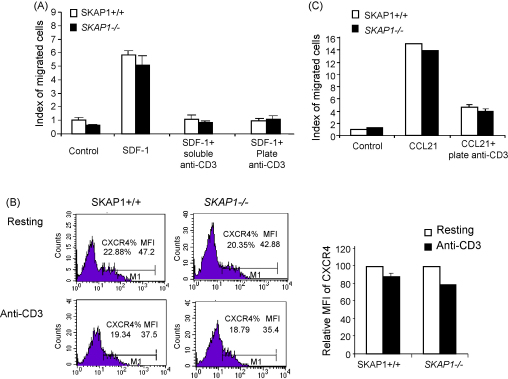
Fig. 3.
Anti-CD3 stimulation induces ‘stop-signal’ to inhibit SDF-1 or CCL21 induced cell migration in SKAP1−/− T-cells. Panel A: The transwell membrane was coated with anti-CD3 (5 μg/ml) in the presence or absence of ICAM-1 (5 μg/ml) for 2 h. After washing once with PBS, WT cells and SKAP1−/− T-cells were seeded to the upper wells and SDF-1 (100 ng/ml) was added to the bottom wells. The number of cells migrated over a 2 h period of exposure to medium alone, SDF-1 alone, or a combination of both SDF-1 and 2C11, were collected and counted. The P values are 0.0254 (Medium alone), 0.0877 (SDF-1), 0.1546 (SDF-1 + anti-CD3). Panel B: Fresh or anti-CD3 stimulated WT or SKAP1 KO CD4+ T-cells were stained with FITC-conjugated anti-CXCR4 and analyzed by FACS. The percentage of CXCR4 positive cells and the mean fluorescence intensity of CXCR4 staining were provided in the FACS profiles. The histogram shows the relative CXCR4 expression levels in anti-CD3 stimulated cells compared to those in resting cells. Panel C: SKAP1+/+ cells and SKAP1−/− T-cells were seeded in the upper wells and CCL21 (100 ng/ml) was added to the bottom wells. Some samples were stimulated with anti-CD3 as described in (A). The number of cells migrated over a 4-h period of exposure were collected and counted. P > 0.05 when compared SKAP1−/− cells to SKAP1+/+ cells in response to CCL21.