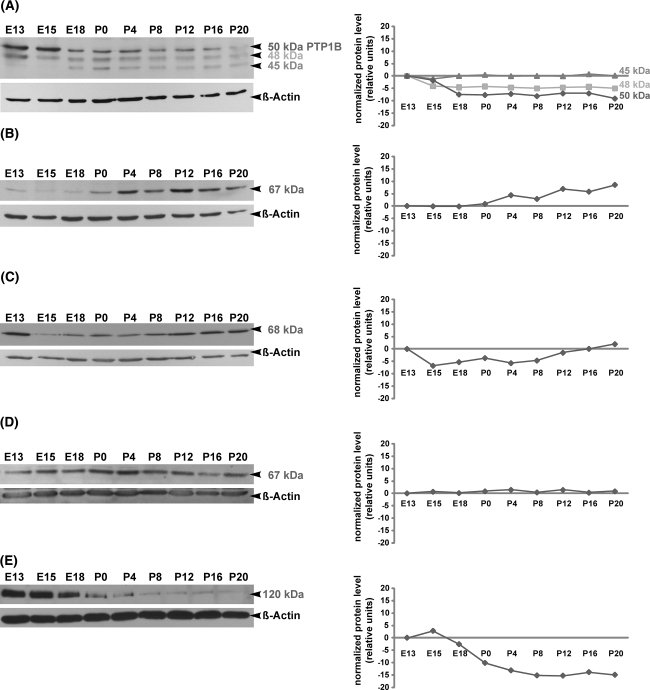
Fig. 2.
Temporal regulation of intracellular PTP proteins as revealed by Western blot analysis in the developing mouse superior colliculus. Protein lysates of superior colliculus tissues from E13, E15, E18, P0, P4, P8, P12, P16 and P20 were separated by SDS-PAGE, and immunoblotted with TC-PTP (a), PTP1C (b), PTP1D (c), PTP-MEG2 (d) and PTP-PEST (e) antibodies, respectively. Molecular weights of intracellular PTP proteins are indicated on the right. All blots were reprobed for β-actin to demonstrate the equal protein loading. Changes in the relative protein expression (normalized protein level) of each PTP protein are shown in the graphical representations. In the case of PTP1C, PTP1D, PTP-MEG2 and PTP-PEST each PTP antibody recognized only one band, which corresponded to the predicted molecular weights of these PTPs. However, the TC-PTP antibody recognized three protein bands at 45, 48 and ~50 kDa (Fig. 2a). The 48 kDa and 45 kDa bands correspond to the previously described two TC-PTP human isoforms, whereas the 50 kDa band corresponds most probably to the intracellular protein tyrosine phosphatase PTP1B. Note that proteins of the investigated intracellular PTPs are differentially regulated during superior collicular development