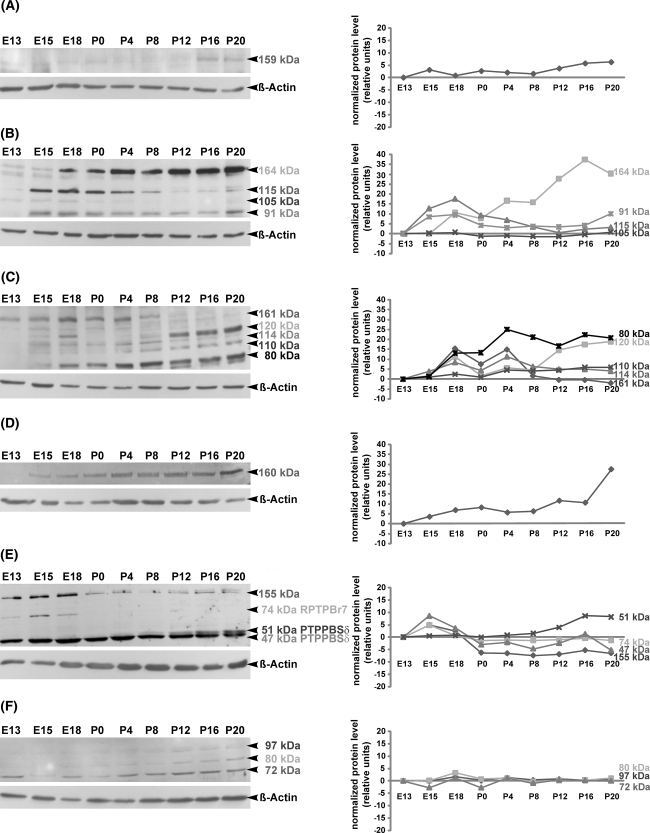
Fig. 3.
Temporal regulation of RPTP proteins as revealed by Western blot analysis in the developing mouse superior colliculus. Protein lysates of superior colliculus tissue from E13, E15, E18, P0, P4, P8, P12, P16 and P20 were separated by SDS-PAGE and immunoblotted with RPTPJ (a), RPTPκ (b), RPTPγ (c), RPTPε (d), RPTPRR (e) and RPTPσ (f) antibodies, respectively. Molecular weights of RPTP proteins are indicated on the right. All blots were reprobed for β-actin to demonstrate the equal protein loading. Changes in the relative protein expression (normalized protein level) of each RPTP protein are shown in the graphical representations. RPTPJ (a) and RPTPε (d) proteins are detected with appropriate antibodies each as single bands at 159 and 160 kDa, respectively. Immunoblots of RPTPκ (b), RPTPγ (c) and RPTPσ (f) reveal several bands, which correspond to the integral proteins and their derived cleavage products. The anti-RPTPκ antibody detects four bands: 164 kDa (full protein) and its 115, 105 and 91 kDa cleavage products (b). The anti-RPTPγ antibody recognizes five bands (c): 161 kDa (full protein) and its 120, 114, 110 and 80 kDa cleavage products. As shown in (f), the antibody against RPTPσ recognizes only the cleavage products at 97, 80 and 72 kDa, whereas the full protein RPTPσ (168 kDa) could not be detected. RPTPRR antibody detects three bands, which correspond to the alternatively spliced receptor-like isoforms of the RPTPRR gene (74 kDa RPTPBr7, 51 kDa PTPPBSδ (+) and 47 kDa PTPPBSδ (−)). An additional 155 kDa band detected by anti-RPTPRR might represent the glycosylated form of one of the receptor-like isoforms (e). Note that the investigated RPTPs and their products (either cleavage products or alternatively spliced isoforms) are differentially regulated during collicular development, providing an evidence for differential regulation at the level of posttranslational modification through proteolytic cleavage or alternative splicing