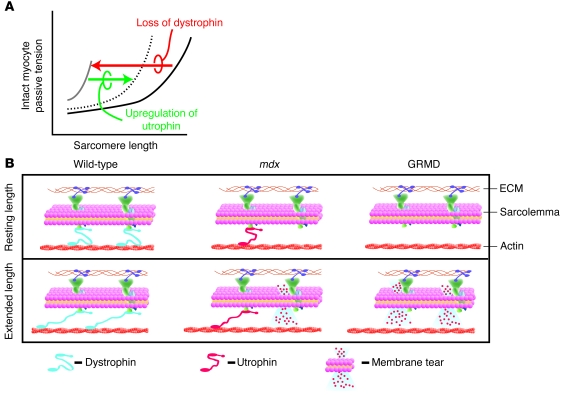
Figure 10. Model of the cellular basis for severe cardiomyopathy in GRMD animals.
(A) A schematic of membane-intact cardiac myocyte passive tension-extension curves. In the presence of dystrophin, myocytes show compliance throughout the working sarcomere length range (black line). In the absence of dystrophin, the cardiac myocytes become very noncompliant and resistant to passive extensions (gray line), as seen in GRMD myocytes. Upregulation of utrophin partially restores the passive extension-tension properties of the dystrophin-deficient myocytes (dotted line), as seen in the mdx myocytes. (B) Cellular model depicting the normal mechanical stabilizing function of dystrophin (blue) in wild-type animals. In wild-type cardiac myocytes, upon passive cell extension (lower scheme), dystrophin protects the membrane from damage. In the mdx myocytes, dystrophin is absent, but utrophin (red) is upregulated and partially replaces the mechanical buffering properties of dystrophin. This provides some, but not full protection of the membrane from mechanical damage. Hence, with cell extension, there is some membrane damage. In the dystrophin-deficient dog myocytes (GRMD), utrophin is not upregulated. Thus, upon cell extension, there is more damage to the membrane.