Abstract
Petri disease and esca are very destructive grapevine decline diseases that occur in most countries where grapevine (Vitis vinifera) is cultivated. Phaeoacremonium species are among the principal hyphomycetes associated with symptoms of the two diseases, producing a range of enzymes and phytotoxic metabolites. The present study compared the phylogeny of a global collection of 118 Phaeoacremonium isolates from grapevines, in order to gain a better understanding of their involvement in Petri disease and esca. Phylogenetic analyses of combined DNA sequence datasets of actin and β-tubulin genes revealed the presence of 13 species of Phaeoacremonium isolated from esca diseased grapevines. Phaeoacremonium aleophilum was the most frequently isolated species with an incidence up to 80 % of all isolates investigated. Species previously described mainly as human pathogenic species, namely Pm. alvesii, Pm. griseorubrum and Pm. rubrigenum are newly reported on grapevine from Turkey, Italy and Croatia, respectively. Phaeoacremonium viticola and Pm. scotyli represent new records for Italy, as well as Pm. mortoniae for Hungary and Croatia. In addition, four new species of Phaeoacremonium, namely Pm. croatiense, Pm. hungaricum, Pm. sicilianum and Pm. tuscanum are newly described from grapevine based on morphology, cultural characteristics, as well as molecular phylogeny.
Keywords: actin, β-tubulin, esca, morphology, Phaeoacremonium, phylogeny
INTRODUCTION
Phaeoacremonium species are well-known vascular plant pathogens causing wilt and dieback of woody hosts. In grapevine, the two principal diseases in which they are involved are Petri disease and esca, the latter of which comprises young esca and esca proper according to the nomenclature of esca diseases proposed by Graniti et al. (2000). Petri disease causes stunted growth and dieback of young grapevines. It often occurs in 1–5 yr old grapevines and causes significant losses in newly planted vineyards (Mugnai et al. 1999, Morton 2000, Pascoe & Cottral 2000, Edwards & Pascoe 2004, Surico et al. 2006). Internal symptoms can normally be seen when transverse or longitudinal cuts are made in the rootstock. These include black spots and dark brown to black streaking of the xylem tissues. Esca can be typically identified by various types of internal wood deterioration as well as symptoms on leaves and berries. Vines with typical symptoms on the leaves show interveinal areas of chlorotic tissue that turn yellow-brown or red-brown and finally necrotic, an appearance that can also be described as ‘tiger stripes’ (Larignon & Dubos 1997, Mugnai et al. 1999, Edwards et al. 2001, Calzarano & Di Marco 2007) (Fig. 1). In the USA, esca has been referred to as ‘black measles’ because of the small, dark-brown to purple spots that can develop on the berries (Fig. 2) (Vasquez et al. 2007). When a transverse cut is made in the trunk and main shoots, black spots (black streaking in longitudinal section) appear in the wood as in the case of Petri disease, but in young esca also pink-brown or dark red-brown areas can be found, occasionally with other wood discolorations (Mugnai et al. 1999). Esca proper (Surico 2001) differs from young esca by the presence of wood decay (Mugnai et al. 1999, Fischer 2002, Surico et al. 2006) (Fig. 3).
Fig. 1.
Symptoms associated with esca of grapevine: chlorosis and necrosis on the leaves showing typical ‘tiger-like’ pattern.
Fig. 2.
Symptoms associated with esca of grapevine: black measles on the berries.
Fig. 3.
Typical wood symptoms in a vine affected by esca: brown-red wood, black streaking, and central white decay.
Foliar and fruit symptoms do not necessarily appear on the same diseased plant every year (Mugnai et al. 1999, Marchi et al. 2006), and often infected vines remain asymptomatic (Surico et al. 2006). In severe cases ‘apoplexy’ can occur when vines or vine-parts suddenly wilt during hot, dry conditions in the summer.
Fungi that have been associated with esca symptoms in Europe include the wood-rotting basidiomycete Fomitiporia mediterranea, and occasionally Stereum hirsutum (Larignon & Dubos 1997, Fischer 2006), while Phaeomoniella chlamydospora and Phaeoacremonium aleophilum are the principal hyphomycetes associated with black streaking and brown-red wood (Larignon & Dubos 1997, Mugnai et al. 1999, Crous & Gams 2000). It is the combination of these fungi that causes ‘esca proper’, affecting mostly vines older than 15 yr, while vines showing esca foliar symptoms, wood black streaking and necrosis are mainly infected with Phaeomoniella chlamydospora and/or Phaeoacremonium species.
Species of Phaeoacremonium mainly involved in Petri disease and esca are Pm. aleophilum, Pm. angustius, Pm. mortoniae and Pm. parasiticum (Eskalen et al. 2005a, Mostert et al. 2006a, Martin & Cobos 2007), but the degree of involvement of other Phaeoacremonium species remains uncertain. Furthermore, the identity of fungi associated with esca symptoms in many grapevine-growing areas, especially from the area where grapevine has originated, and several isolated regions have not yet been studied, and therefore many elusive aspects remain to be clarified.
The present study investigates the identity of a group of 118 Phaeoacremonium isolates from grapevine, collected mainly from very old vines, in isolated locations in Italy and other countries. Knowledge pertaining to the involvement of Phaeoacremonium species in esca and Petri disease should shed light on the epidemiology of these destructive diseases of grapevine, with the final aim of helping in refining control strategies, since there are no effective curative chemicals for Petri disease and esca.
MATERIAL AND METHODS
Fungal isolates
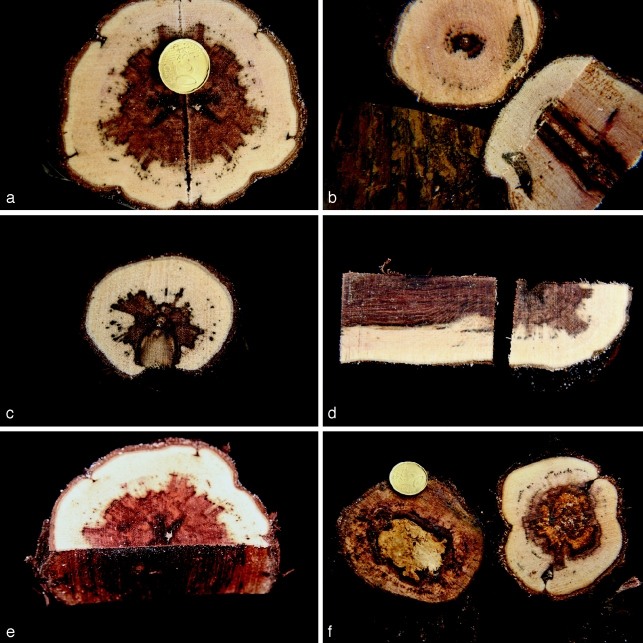
Branches and trunks of Vitis vinifera showing esca symptoms in wood, including brown and black streakings, brown-red wood, necrosis and white rot (Fig. 4) and in some cases also foliar symptoms of esca, were collected from different regions of Italy, primarily from isolated locations and very old vineyards (80–100 yr old), from different vineyards within the same region and from different positions on the same vine. Other strains, collected from different countries (Croatia, Greece, Hungary, Israel, Turkey and the USA), were also investigated in this study.
Fig. 4.
Internal symptoms seen when transversal or longitudinal cuts were made in the trunk or cordon of vines used for fungal isolation. a, b. Black spots and dark brown to black streaking of the xylem tissues; c. cross section showing sectorial necrosis; d. longitudinal section showing wood discoloration; e. central brown-red necrosis; f. cross section showing a central white rot surrounded by brown-red wood.
Trunk and shoots of diseased grapevines were cut into disks and the surface was sterilised. Small pieces of tissue were cut from just below the surface, around and in the darkened vascular tissues, and plated onto malt extract agar (MEA; 2 % malt extract, Oxoid Ltd., Basingstoke, Hampshire, England; 1.5 % agar, Difco, Detroit, Michigan, USA) and incubated at 25 °C in the dark for 2–3 wk until cultures sporulated. Single conidial isolations were established from emerging colonies identified as species of Phaeoacremonium. Isolates were maintained at the Dipartimento di Biotecnologie Agrarie, Sezione di Patologia Vegetale, University of Florence, and representative strains lodged at the CBS Fungal Biodiversity Centre, Utrecht, Netherlands. Isolates used for morphological and sequence analysis are presented in Table 1.
Table 1.
Names, GenBank accession numbers and collection details of Phaeoacremonium isolates studied. Phaeoacremonium aleophilum sequence types based on ACT and TUB respectively are indicated between round brackets (superscript in isolate number).
| Phaeoacremonium species | Isolate number | Location | GenBank accession numbers |
|
|---|---|---|---|---|
| ACT | β-tubulin | |||
| Pm. aleophilum | 4.ss2Pal (1/1) | Tuscany, Italy | EU863496 | EU863464 |
| 146Pal (1/1) | Abruzzo, Italy | |||
| 32Pal (1/1) | Marche, Italy | |||
| 59Pal, 69Pal, 75Pal, 76.ss1Pal (1/1) | Sicily, Italy | |||
| 4ss1Pal, 23Pal (1/1) | Tuscany, Italy | |||
| 140Pal (2/1) | Friuli-Venezia Giulia, Italy | |||
| 33Pal (2/1) | Marche, Italy | |||
| 38Pal, 39Pal (2/1) | Sardinia, Italy | |||
| 44ss1Pal, 51Pal, 52a.ss1Pal, 52ass2 Pal, 52-bPal, 53Pal, 64Pal, 70Pal, 71Pal, 72Pal, 77Pal, 80Pal (2/1) | ||||
| Sicily, Italy | ||||
| 30Pal (2/1) | Trentino-Alto Adige, Italy | |||
| 17Pal, 20Pal (2/1) | Tuscany, Italy | |||
| 143ss2Pal (2/1) | Umbria, Italy | |||
| 124Pal, 125ss1Pal, 126Pal, 127Pal (2/1) | Turkey | |||
| 60Pal, 61Pal, 62Pal, 73Pal, 74Pal, 78Pal, 79Pal (3/1) | Sicily, Italy | |||
| 142Pal (3/1) | Tuscany, Italy | |||
| 145Pal (4/1) | Abruzzo, Italy | |||
| 148Pal (4/1) | Apulia, Italy | |||
| 100Pal, 101Pal, 103Pal, 104Pal (4/1) | Hungary | |||
| 130Pal, 131Pal, 133Pal (6/1) | Israel | |||
| 81Pal (2/2) | Sicily, Italy | EU863497 | EU863465 | |
| 168Pal (2/3) | Trentino-Alto Adige, Italy | EU863498 | EU863466 | |
| 56Pal (1/3) | Sicily, Italy | |||
| 31Pal (1/3) | Trentino-Alto Adige, Italy | |||
| 158Pal (1/3) | Tuscany, Italy | |||
| 139Pal (2/3) | Lombardy, Italy | |||
| 47Pal, 57Pal, 58Pal, 65Pal, 66Pal, 67Pal, 68Pal (2/3) | Sicily, Italy | |||
| 167Pal, 171Pal (2/3) | Trentino-Alto Adige, Italy | |||
| 13Pal, 14Pal, 22Pal, 24Pal, 25Pal, 28Pal, 152Pal, 153Pal (2/3) | Tuscany, Italy | |||
| 115Pal, 116Pal (2/3) | Croatia | |||
| 120Pal, 121Pal, 122Pal, 123Pal, 128Pal, 129Pal (2/3) | Turkey | |||
| 159Pal, 161Pal (2/3) | U.S.A | |||
| 137Pal, 138ss1Pal (3/4) | Lombardy, Italy | EU863500 | EU863468 | |
| 156Pal (5/4) | Tuscany, Italy | EU863499 | EU863467 | |
| 84Pal, 85Pal (1/4) | Greece | |||
| 117Pal, 118Pal (2/4) | Croatia | |||
| 98Pal (3/5) | Hungary | EU863501 | EU863469 | |
| 21Pal (4/6) | Tuscany, Italy | EU863502 | EU863470 | |
| 144Pal (1/7) | Abruzzo, Italy | EU863503 | EU863471 | |
| 132Pal (6/8) | Israel | EU863504 | EU863472 | |
| Pm. alvesii | 125ss2 Pal | Turkey | EU883991 | EU883990 |
| Pm. croatiense sp.nov. | CBS 123037 | Croatia | EU863514 | EU863482 |
| Pm. hungaricum sp.nov. | CBS 123036 | Hungary | EU863515 | EU863483 |
| Pm. iranianum | 2Pal | Tuscany, Italy | EU863491 | EU863459 |
| 3Pal | Tuscany, Italy | EU863492 | EU863460 | |
| 6Pal | Tuscany, Italy | EU863493 | EU863461 | |
| 7Pal | Tuscany, Italy | EU863494 | EU863462 | |
| 163Pal | Piedmont, Italy | EU863495 | EU863463 | |
| Pm. griseorubrum | 42Pal | Sicily, Italy | EU863517 | EU863485 |
| Pm. mortoniae | 110bss1Pal | Hungary | EU863507 | EU863475 |
| 110.ss2Pal | Hungary | EU863508 | EU863476 | |
| 111Pal | Hungary | EU863509 | EU863477 | |
| 112ss1Pal | Hungary | EU863510 | EU863478 | |
| 110bss2Pal | Hungary | EU863511 | EU863479 | |
| 114Pal | Hungary | EU863512 | EU863480 | |
| 94Pal | Croatia | EU863513 | EU863481 | |
| Pm. parasiticum | 40ss2Pal | Sicily, Italy | EU863519 | EU863487 |
| Pm. rubigenum | CBS 123038 | Croatia | EU863516 | EU863484 |
| Pm. scolyti | 109Pal | Tuscany, Italy | EU863518 | EU863486 |
| Pm. sicilianum sp.nov. | CBS 123034 | Sicily, Italy | EU863520 | EU863488 |
| CBS 123035 | Sicily, Italy | EU863521 | EU863489 | |
| Pm. tuscanum sp.nov. | CBS 123033 | Tuscany, Italy | EU863490 | EU863458 |
| Pm. viticola | 40ss1Pal | Sicily, Italy | EU863505 | EU863473 |
| 41Pal | Sicily, Italy | EU863506 | EU863474 | |
DNA isolation and amplification
Genomic DNA was extracted from 118 strains identified as Phaeoacremonium using approximately 300 mg mycelium with the UltraClean™ Microbial DNA Kit (MO Bio, Carlsbad, CA, USA) according to the manufacturer’s instructions. Approximately 600 bp of the 5′ end of the β-tubulin (TUB) and approximately 300 bp of the 5′ end of the actin (ACT) genes were amplified for the strains identified as Phaeoacremonium as described by Mostert et al. (2006b) using primer sets T1 (O’Donnell & Cigelnik 1997) and Bt2b (Glass & Donaldson 1995) and ACT-512F and ACT-783R (Carbone & Kohn 1999), respectively.
Amplicons were sequenced using both PCR primers with a BigDye Terminator Cycle Sequencing Kit v. 3.1 (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s instructions, and sequences were analysed on an ABI Prism 3700 DNA Sequencer (Perkin-Elmer, Norwalk, Foster City, CA, USA). A consensus sequence was computed from the forward and reverse sequences with the SeqMan from the Lasergene package (DNA star, Madison, WI, USA).
Phylogenetic analysis
Sequences were manually aligned using Sequence Alignment Editor v. 2.0a11 (Se-Al; Rambaut 2002) by inserting gaps, and additional reference sequences were obtained from GenBank and added to the alignment. The TUB and ACT alignments were concatenated to make it possible to perform combined analyses. Phylogenetic analyses of the aligned sequence data were performed with PAUP (Phylogenetic Analysis Using Parsimony) v. 4.0b10 (Swofford 2003) and consisted of neighbour-joining analyses with the uncorrected (‘p’), the Kimura 2-parameter and the HKY85 substitution models. Alignment gaps were treated as missing data and all characters were unordered and of equal weight. Any ties were broken randomly when encountered. For parsimony analyses, alignment gaps were treated as a fifth character state and all characters were unordered and of equal weight. Maximum parsimony analysis was performed using the heuristic search option with 100 random simple taxa additions and tree bisection and reconstruction (TBR) as the branch-swapping algorithm. Branches of zero length were collapsed and all multiple, equally parsimonious trees were saved. The robustness of the trees obtained was evaluated by 1 000 bootstrap replications (Hillis & Bull 1993). Tree length (TL), consistency index (CI), retention index (RI) and rescaled consistency index (RC) were calculated and the resulting trees were printed with TreeView v. 1.6.6 (Page 1996). New sequences were lodged in GenBank and the alignment and phylogenetic tree in TreeBASE (www.treebase.org). Pleurostomophora richardsiae (CBS 270.33; GenBank ACT = AY579271, TUB = AY579334) was used as outgroup in the phylogenetic analyses.
Morphology
Morphological characters used in distinguishing species included conidiophore morphology, phialide type and shape, size of hyphal warts, and to a lesser extent, conidial size and shape. Cultural characters that were investigated included the colour of colonies on MEA, the production of yellow pigment on potato-dextrose agar (PDA; 3.9 % potato-dextrose agar, Difco) and oat meal agar (OA, 30 g oats; 8 g Roko agar, La Coruña, Spain; 1 000 mL water) (Gams et al. 2007), the growth rate of colonies at 25 °C and the maximum temperature for growth in vitro.
Microscopic observations were made from aerial mycelium of colonies cultivated on MEA or by using the transparent tape or slide culture technique, as respectively explained by Schubert et al. (2007) and Arzanlou et al. (2007). Photos were captured by means of a Nikon camera system (Digital Sight DS-5M, Nikon Corporation, Japan). Structures were mounted in lactic acid, and 30 measurements (× 1 000 magnification) were determined. The 5th and 95th percentiles were defined for all measurements with the extremes given in parentheses.
Cardinal temperatures for growth were determined by incubating inoculated MEA plates in the dark at temperatures ranging from 6 to 40 °C. Radial growth was measured after 8 d at 25 °C. Colony colours were defined after 16 d from the same plates according to the colour charts of Rayner (1970).
RESULTS
Phylogenetic analyses
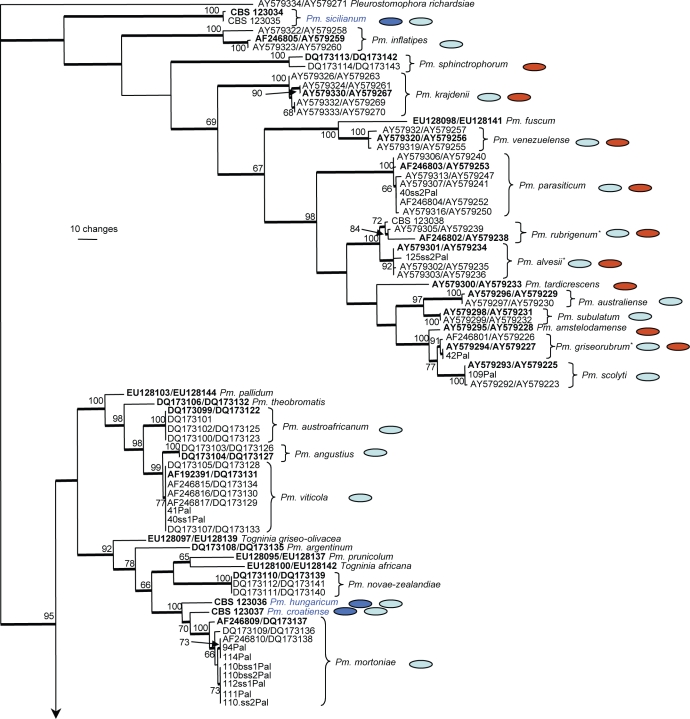
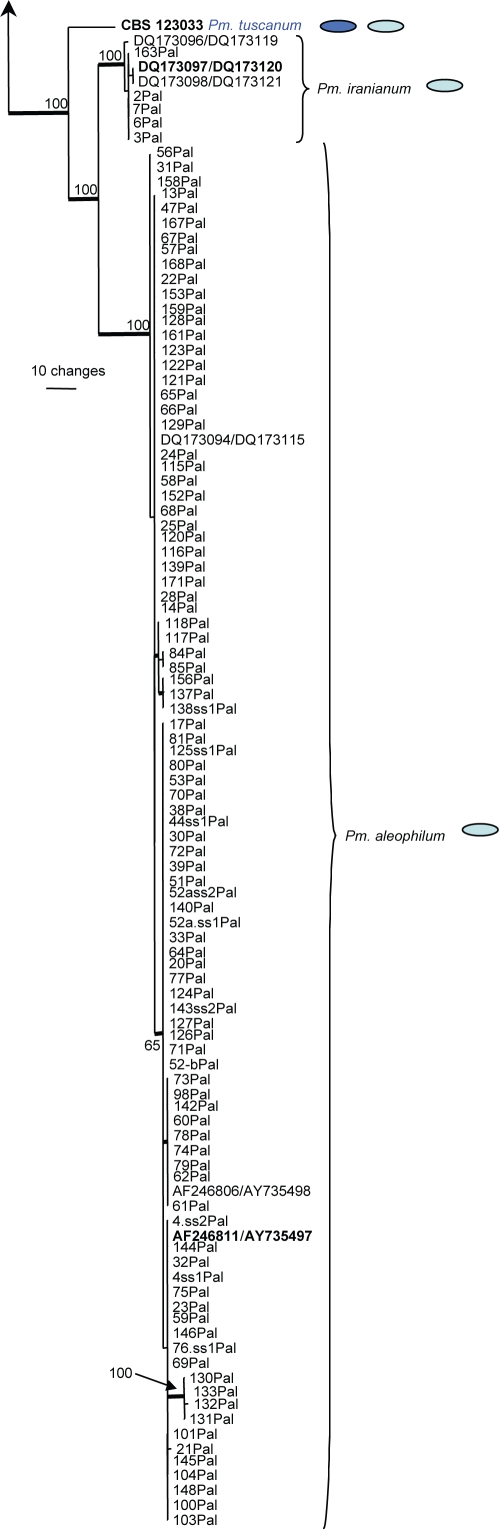
The combined alignment consisted of 184 sequences including the outgroup sequence and included 473 characters and alignment gaps (number of included characters: TUB = 266 and ACT = 207) that were subjected to the phylogenetic analyses. Of these, 275 were parsimony informative, 47 were variable and parsimony uninformative and 151 were constant. The small number of characters included for the TUB is due to the inclusion of GenBank accession AF192391, which represents the TUB sequence of the type strain of Pm. viticola and which is missing more than 250 characters on the 5′ end when compared to the other sequences in the alignment. Parsimony analyses yielded 317 equally most parsimonious trees that mainly differed in the order of taxa at the terminal nodes; one of the trees is presented in Fig. 5 (TL = 1304; CI = 0.508; RI = 0.922; RC = 0.468; HI = 0.492). Neighbour-joining analysis using the three substitution models on the sequence data yielded trees with similar topology and bootstrap values. The phylogenetic tree clustered some isolates obtained in this study with previously published species and indicated that others did not match any sequences available in GenBank. The second group of sequences represents unknown species which are described below.
Fig. 5.
One of 317 most parsimonious trees obtained from heuristic searches of a combined alignment of the TUB and ACT gene sequences (length = 1304 steps, CI = 0.508, RI = 0.922, RC = 0.468, HI = 0.492). Bootstrap support values above 64 % are shown at the nodes. Pleurostomophora richardsiae was used as outgroup. Accessions numbers of sequences obtained from the GenBank nucleotide database are indicated on the tree in the format TUB/ACT. Ex-type strains are emphasised in bold. Names in blue are novel species described in this study.
 New species described in this study;
New species described in this study;  Human pathogenic species;
Human pathogenic species;  Species isolated from grapevine; * Human pathogenic species newly reported from grapevine.
Species isolated from grapevine; * Human pathogenic species newly reported from grapevine.
Analyses of the individual loci did not reveal any significant deviation from the topology obtained from analyses of the combined alignment and 72 equally most parsimonious trees were obtained for both loci (data not shown). For the TUB data, the 266 characters including alignment gaps consisted of 136 parsimony informative, 31 variable and parsimony uninformative and 99 constant characters; for the ACT data the 207 characters including alignment gaps consisted of 139 parsimony informative, 16 variable and parsimony uninformative and 52 constant characters.
Taxonomy
According to DNA sequence analyses and morphological characters, the 118 strains isolated from wood of Vitis vinifera showing Petri disease or esca symptoms could be assigned to 13 different species of Phaeoacremonium. Four taxa proved to be distinct from known species and are described in this study. In addition to the novel species, the morphologically variant form seen in Pm. rubrigenum isolate CBS 123038 was discussed in contrast to the isolates described by Mostert et al. (2006b).
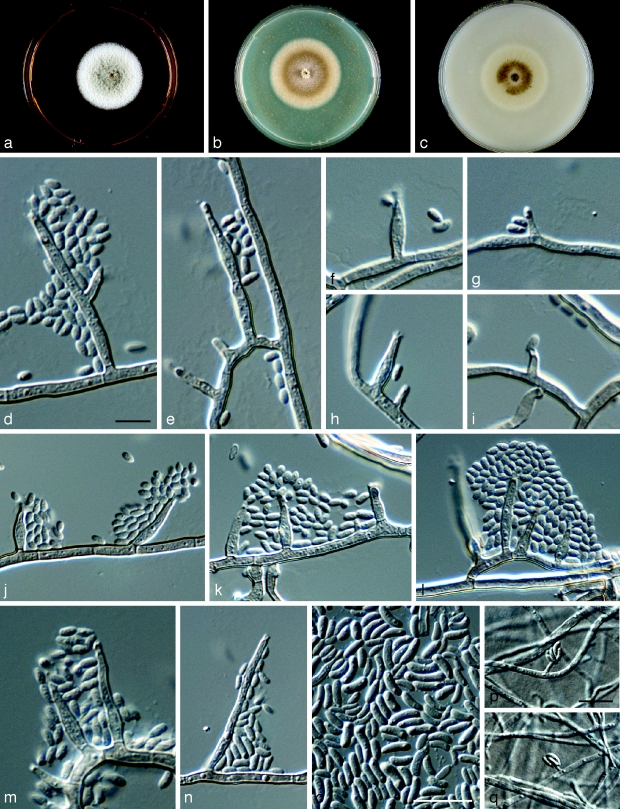
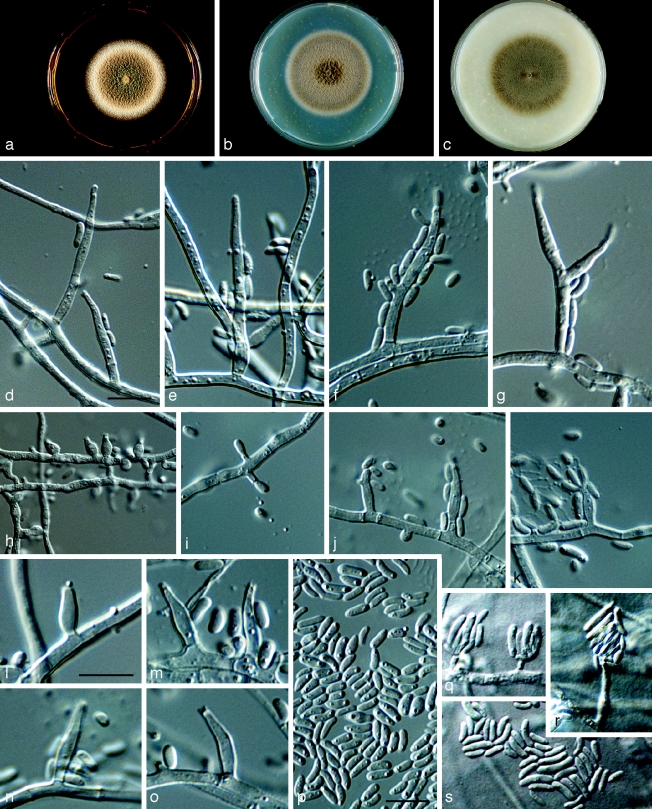
Phaeoacremonium croatiense Essakhi, Mugnai, Surico & Crous, sp. nov. — MycoBank MB506947; Fig. 6
Fig. 6.
Phaeoacremonium croatiense. a–c. Sixteen days old colonies on 2 % MEA (a), PDA (b) and OA (c). — d–o. Aerial structures on 2 % MEA; d, e. conidiophores; f–i. type I phialide; j–l. type II phialide; m, n. type III phialide; o. conidia. — p–q. Structures on the surface of and in 2 % MEA: adelophialides with conidia; all from CBS H-20120 (holotype); d–q: DIC. — Scale bars: d–p = 10 μm; scale bar for d applies to e–n and q.
Phaeoacremonio mortoniae phylogenetice simile, sed coloniis olivaceo-griseis in agaro MEA, sine pigmento flavido in agaro OA.
Etymology. Named after Croatia, where this species was collected.
Aerial structures in vitro on MEA: Mycelium consisting of branched, septate hyphae that occur singly or in bundles of up to 4; tuberculate with warts up to 0.5 μm wide, subhyaline to pale brown, smooth to verruculose, 2–3 μm wide. Conidiophores mostly of medium length, usually unbranched, arising from aerial or submerged hyphae, erect to flexuous, up to 5-septate, often ending in a single terminal phialide, subhyaline to pale brown, paler towards the tip, smooth to verruculose, (10–)16–23(−48) (av. 19) μm long and (2–)2.5(−3) (av. 2.5) μm wide. Phialides terminal or lateral, mostly monophialidic, smooth to verruculose, hyaline to subhyaline, collarettes, 1.5–2 μm long, 1–1.5 μm wide; type I phialides predominant, mostly cylindrical to subcylindrical, or elongated ampulliform, attenuated at the base, (6–)11–13(−15) × (1.5–)2.5(−3) (av. 12 × 2.5) μm; type II phialides mostly subulate, some navicular, tapering towards the apex, (10–)15–19(−20) × (2–)2.5(−3) (av. 17 × 2.5) μm; type III phialides subcylindrical, subulate, (20–)23–27(−28) × 2(−2.5) (av. 24 × 2) μm. Conidia hyaline, mostly subcylindrical or allantoid, some cylindrical or ellipsoidal, (2–)3–4.5(−7) × (1–)1.5(−2) (av. 4 ×1.5) μm, L/W = 2.6. On surface or submerged in the agar: Phialides hyaline, mostly cylindrical to subcylindrical, (2–)5–8(−12) × (1.5–)2(−3) (av. 7 × 2) μm. Conidia hyaline, subcylindrical, or allantoid, (4–)6–7(−9) × (1–)2 (av. 6.5 × 2) μm, L/W = 3.25.
Cultural characteristics — Colonies reaching a radius of 10 mm after 8 d at 25 °C. Minimum temperature for growth 12 °C, optimum 27 °C, maximum 33 °C. Colonies on MEA flat, cottony, with entire margin; after 16 d, pale olivaceous-grey to whitish above, orange to yellowish white in reverse. Colonies on PDA flat, short woolly to felty, with entire edge, after 16 d, colonies smoke-grey to pale grey-olivaceous above, white towards the margin above, brownish grey in reverse. Colonies on OA flat, felty, with entire margin, after 16 d, grey-olivaceous to whitish towards the edge above, pale olivaceous-grey, yellowish white towards the edge in reverse.
Substrate — Vitis vinifera.
Known distribution — Croatia.
Specimen examined. Croatia, Moslavina, Voloder, isolated from Vitis vinifera (cv. Škrlet) cutting showing necrosis and black streakings, June 2007, B. Cvjetković, holotype CBS H-20120, culture ex-type CBS 123037.
Notes — DNA sequence analyses revealed Pm. croatiense to be closely related to Pm. mortoniae. It can, however, be distinguished based on its olivaceous-grey colonies on MEA, as well as by the absence of yellow pigment production on OA.
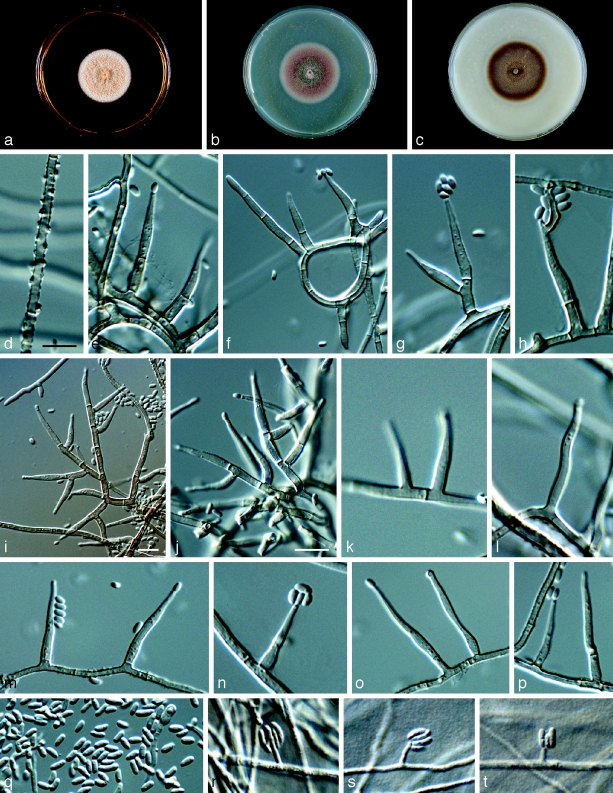
Phaeoacremonium hungaricum Essakhi, Mugnai, Surico & Crous, sp. nov. — MycoBank MB506948; Fig. 7
Fig. 7.
Phaeoacremonium hungaricum. a–c. Sixteen days old colonies on 2 % MEA (a), PDA (b) and OA (c). — d–p. Aerial structures on 2 % MEA. d–g. conidiophores; h–k. type I phialide; i–o. type II phialide; p. conidia. — q–s. Structures on the surface of and in 2 % MEA; q–r. adelophialides with conidia; s. conidia; all from CBS H-20119 (holotype); d–s: DIC. — Scale bars: d–s = 10 μm; scale bar for d applies to e–k and q–s; bar for l applies to m–o.
Phaeoacremonio mortoniae phylogenetice simile, sed structuris coremioidibus fertilibus in agaro MEA et phialidibus plerumque typi II.
Etymology. Named after Hungary, where this species was collected.
Aerial structures in vitro on MEA: Mycelium composed of branched, septate hyphae that occur singly or in bundles of up to 14, subhyaline to medium brown, smooth, occasionally verruculose, 1–3.5 μm wide. Conidiophores mostly short, usually unbranched, arising from aerial or submerged hyphae, erect, simple, up to 2-septate, often ending in a single terminal phialide, subhyaline to pale brown, paler towards the tip, smooth to verruculose, (20–)26–30(−36) (av. 27) μm long and (2–)2.5(−3) (av. 2.5) μm wide. Phialides terminal or lateral, mostly monophialidic, smooth to verruculose, mostly subhyaline, sometimes pale brown, collarettes, 1 μm long, 1.5 μm wide; type I phialides most predominant, elongated ampulliform, attenuated at the base, or constricted, some cylindrical, (3–)7–12(−15) × (1–)2.5(−3) (av. 7 × 2.5) μm; type II phialides navicular or subulate, subcylindrical, tapering towards the apex, (9–)12–15(−20) × (1.5–)2.5(−3) (av. 13 × 2.5) μm. Conidia hyaline, mostly, subcylindrical or cylindrical, often allantoid, (3–)4–5(−6) ×(1.5–)2 (av. 4.5 × 2) μm, L/W = 2.25. On surface or submerged in the agar: Phialides hyaline, cylindrical to subcylindrical, occasionally navicular, (2–)7–11(−15) × (1.5–)2(−3) (av. 9 × 2) μm. Conidia hyaline, cylindrical, subcylindrical or allantoid (3–)5–7.5(−12) × (1–)2.5(−3) (av. 6.5 × 2) μm, L/W = 3.75.
Cultural characteristics — Colonies reaching a radius of 10 mm after 8 d at 25 °C. Minimum temperature for growth 10 °C, optimum 27 °C, maximum 33 °C. Colonies on MEA flat, woolly, with entire margin; after 16 d, whitish yellow to whitish above, dark brown to pale orange in reverse. Colonies on PDA flat, felty, with entire edge, after 16 d, colonies beige to whitish grey-olivaceous, white towards the margin above. Colonies on OA flat, felty, with entire margin, after 16 d, greenish olivaceous to white towards the edge; greenish olivaceous above, olivaceous-grey in reverse.
Substrate — Vitis vinifera.
Known distribution — Hungary.
Specimen examined. Hungary, Mád, Tokaj, isolated from Vitis vinifera (cv. Hárslevelű) showing external esca symptoms, wood necrosis and black streaking, Feb. 2007, B.T. Dula, holotype CBS H-20119, culture ex-type CBS 123036.
Notes — Phylogenetically, this species clusters as a sister clade to Pm. mortoniae. However, it can be distinguished by its conidiophores which are mostly reduced to phialides. The aerial mycelium has an abundant number of phialides, which are elongated ampulliform in shape.
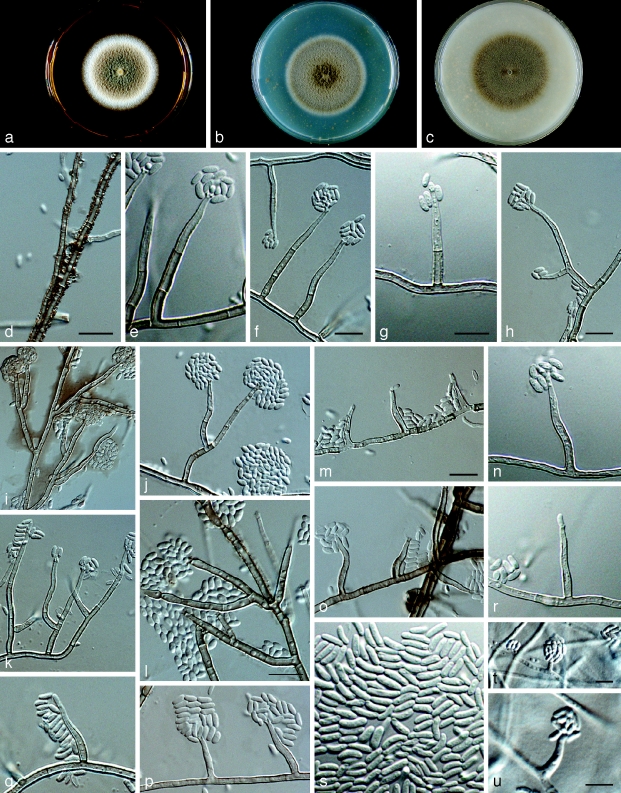
Phaeoacremonium rubrigenum W. Gams, Crous & M.J. Wingf., Mycologia 88: 795. 1996. — Fig. 8
Fig. 8.
Phaeoacremonium rubrigenum. a–c. Sixteen days old colonies on 2 % MEA (a), PDA (b) and OA (c). — d–q. Aerial structures on 2 % MEA; d. mycelium showing prominent exudate droplets observed as warts; e–h. single conidiophores; i, j. branched conidiophores; k, l. type I phialide; m, n. type II phialide; o, p. type III phialide; q. conidia. — r–t. Structures on the surface of and in 2 % MEA: adelophialides with conidia; all from H-20121 (holotype); d–t: DIC. — Scale bars: d = 10 μm; scale bar for d applies to i–k and k–t.
Aerial structures in vitro on MEA: Mycelium consisting of branched, septate hyphae that occur singly or in bundles of up to 8; tuberculate with warts up to 1 μm wide, subhyaline to pale brown, smooth to verrucose, 1.5–2.5 μm wide. Conidiophores mostly of medium length, arising from aerial or submerged hyphae, branched, occasionally unbranched, each branched conidiophores often ending in a single terminal phialide, occasionally also with lateral phialides; erect, up to 6-septate, subhyaline to pale brown, paler towards the tip, smooth to verrucose, (18–)27–32(−48) (av. 29) μm long and (1.5–)2.5(−3) (av. 2.5) μm wide. Phialides terminal or lateral, mostly monophialidic, smooth to verruculose, pale brown to subhyaline; collarettes, 2–3 μm long, 1–1.5 μm wide; type II phialides most common, type I phialides subcylindrical, or elongated ampulliform, attenuated at the base, or constricted, (5–)8–11(−15) × (1–)2(−2.5) (av. 9 × 2) μm; type II phialides mostly subulate, some navicular, tapering towards the apex, (10–)15–18(−20) × (2–)2.5(−3) (av. 17 × 2.5) μm; type III phialides rarely present, subulate, tapering towards the apex, (25–)26(−28) × (2–)2.5(−3) (av. 26 × 2.5) μm. Conidia hyaline, mostly ellipsoidal, some cylindrical, (3–)3.5–4(−6.5) × (1–)1.5(−2) (av. 4 × 1.5) μm, L/W = 2.6. On surface or submerged in the agar: Phialides hyaline, mostly navicular, tapering towards the apex, some cylindrical, (2–)5–8(−12) × (1.5–)2(−3) (av. 9 × 2) μm. Conidia hyaline, allantoid or subcylindrical, (3–)4.5–5.5(−8) × 1.5(−2) (av. 5 × 1.5) μm, L/W = 3.3.
Cultural characteristics — Colonies reaching a radius of 9 mm after 8 d at 25 °C. Minimum temperature for growth 12 °C, optimum 27 °C, maximum 33 °C. Colonies on MEA flat, cottony to woolly, with entire margin; after 16 d, beige, whitish towards the margin above, brown to orange in reverse. Colonies on PDA flat, appressed, woolly to powdery, with entire edge, after 16 d, colonies brown to vinaceous-white towards the margin above, brown to violet in reverse. Colonies on OA flat, felty, with entire margin, after 16 d, brown to greyish sepia above, pale purplish grey in reverse.
Substrate — Human, Vitis vinifera.
Known distribution — USA, Croatia.
Specimen examined. Croatia, Šibenik, isolated from Vitis vinifera (cv. Lasina), showing necrosis and black streakings, June 2007, B. Cvjetković, CBS H-20121, culture ex-type CBS 123038.
Notes — Phylogenetically this isolate clusters with other strains of Pm. rubrigenum. It is morphologically different, however, in its predominance of branched conidiophores and in its beige colonies on MEA. In contrast, Mostert et al. (2006b) described colonies of Pm. rubrigenum as having usually unbranched conidiophores and being pink to purplish on MEA.
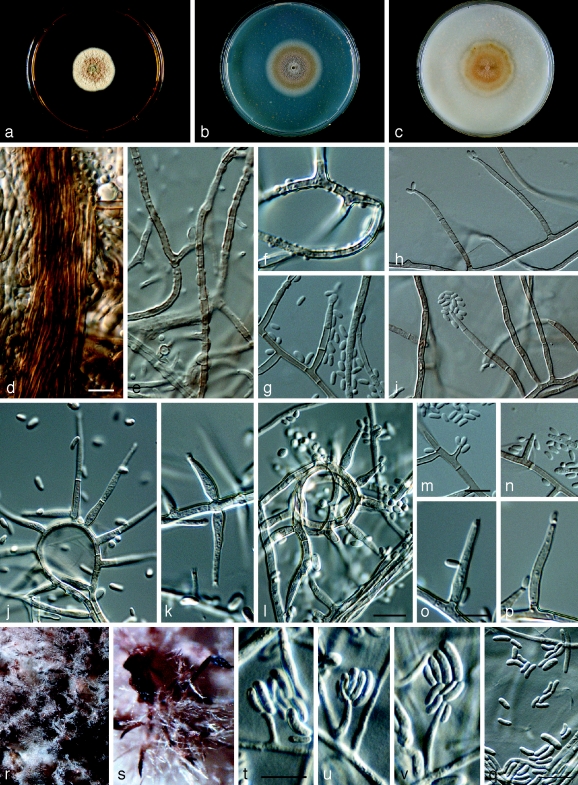
Phaeoacremonium sicilianum Essakhi, Mugnai, Surico & Crous, sp. nov. — MycoBank MB506949; Fig. 9
Fig. 9.
Phaeoacremonium sicilianum. a–c. Sixteen days old colonies on 2 % MEA (a), PDA (b) and OA (c). — d–s. Aerial structures on 2 % MEA; d. mycelium showing prominent exudate droplets observed as warts; e–h. single conidiophores; i–l. branched conidiophores; m–p. type I phialide; q. type II phialide; r. type III phialide; s. conidia. — t–u. Structures on the surface of and in 2 % MEA: adelophialides with conidia; all from CBS H-20118 (holotype); d–u: DIC. — Scale bars: d–u = 10 μm; scale bar for d applies to e and i–k; bar for n applies to o–r.
Phaeoacremonio parasitico et P. inflatipedi simile. Differt a P. parasitico hyphis magnis sine verrucis, et a P. inflatipedi phialidibus plerumque typorum I et II.
Etymology. Named after the island of Sicily, from where the species was collected.
Aerial structures in vitro on MEA: Mycelium consisting of branched, septate hyphae that occur singly or in bundles of up to 5; tuberculate with warts up to 1 μm diam, smooth to verruculose, medium to pale brown, 1.5–3 μm wide. Conidiophores mostly short and branched, occasionally unbranched, arising from aerial or submerged hyphae, erect, up to 4-septate, often bearing a terminal phialide and an additional lateral one, pale brown to subhyaline, paler towards the tip, smooth to verruculose, (15–)22–47(−68) (av. 35) μm long and (1.5–)2.5(−3) (av. 2.5) μm wide. Phialides terminal or lateral, mostly monophialidic, smooth to verruculose, mostly subhyaline, occasionally pale brown, collarettes, 1.5–3 μm long, 1–2 μm wide; type I phialides, cylindrical to subcylindrical, tapering towards the apex and often widened at the base, (4–)9–12(−17) × (1–)2(−3) (av. 9 × 2) μm; type II phialides subulate, subcylindrical, tapering towards the apex, (9–)15–18(−23) × (1.5–)2.5(−3) (av. 18 × 2.5) μm; type III phialides subcylindrical, navicular, (20–)23–27(−28) × 2(−2.5) (av. 25 × 2) μm. Conidia hyaline, mostly allantoid, subcylindrical (3–)4–6(−10) × 1.5–2(−2.5) (av. 5 × 2) μm, L/W = 2.5. On surface or submerged in the agar: Phialides hyaline, cylindrical to subcylindrical, (3–)4–13(−17) × 2.5 (av. 6 × 2) μm. Conidia hyaline, mainly allantoid, some subcylindrical (3.5–)6–8(−11) × 1.5(−2) (av. 7 × 2) μm, L/W = 3.5.
Cultural characteristics — Colonies attained a radius of 12 mm after 8 d at 25 °C. Minimum temperature for growth 15 °C, optimum 27 °C, maximum 33 °C. Colonies on MEA flat, cottony, with entire margin; after 16 d, pale greyish sepia to beige towards the edge above, brown to pale orange in reverse. Colonies on PDA flat, cottony to woolly, with entire edge, after 16 d, colonies pale brown to smoke-grey above, olivaceous-grey in reverse. Colonies on OA flat, felty to powdery, with entire margin; after 16 d, smoke-grey to pale olivaceous above, olivaceous-grey to pale mouse-grey in reverse.
Substrate — Vitis vinifera.
Known distribution — Italy.
Specimen examined. Italy, Sicily, Messina, San Filippo del Mela, isolated from the necrotic margins and brown to black streakings of branches and trunk of very old Vitis vinifera vines showing wood esca symptoms, May 2007, L. Mugnai, holotype CBS H-20118, culture ex-type CBS 123034.
Notes — DNA sequence analysis revealed this species to be basal to other species of Phaeoacremonium. Nevertheless, in terms of morphological characters, Pm. parasiticum and Pm. inflatipes are similar to Pm. sicilianum in the predominance of branched conidiophores. Phaeoacremonium parasiticum is distinct from Pm. sicilianum by virtue of its dark brown hyphae, and large hyphal warts of up to 3 μm diam, while Pm. sicilianum can be distinguished from Pm. inflatipes by the predominance of type I and II phialides, in comparison with the predominance of phialide type III in Pm. inflatipes. Differences in colony colour also distinguish Pm. sicilianum from Pm. inflatipes.
Phaeoacremonium tuscanum Essakhi, Mugnai, Surico & Crous, sp. nov. — MycoBank MB506950; Fig. 10
Fig. 10.
Phaeoacremonium tuscanum. a–c. Sixteen days old colonies on 2 % MEA (a), PDA (b) and OA (c). — d–q. Aerial structures on 2 % MEA; d. mycelium occurring in bundles of up to nine; e, f. mycelium showing prominent exudate droplets observed as warts; g–j. single conidiophores; k, l. type II phialide; m, n. type I phialide; o, p. type III phialide; q. conidia. — r–v. Structures on the surface of and in 2 % MEA; r, s. coremium-like structures; t–v. adelophialides with conidia; all from CBS H-20118 (holotype); r, s: DM; d–q: DIC. — Scale bars: d–v = 10 μm; scale bar for d applies to e–k and p–s; bar for m applies to n; bar for t applies to u–v.
Phaeoacremonio iraniano phylogenetice simile, sed structuris coremioidibus fertilibus in agaro MEA et phialidibus plerumque typi II.
Etymology. Named after Tuscany, Italy, where this fungus was collected.
Aerial structures in vitro on MEA: Mycelium composed of branched, septate hyphae that occur singly or in bundles of up to 22; tuberculate with warts up to 1 μm diam, smooth to verruculose, medium brown to subhyaline and 1.5–2.5 μm wide. Conidiophores mostly short and usually unbranched, occasionally branched, arising from aerial or submerged hyphae, straight, simple, up to 3-septate, usually bearing one terminal phialide, pale brown to subhyaline, paler towards the tip, smooth to verruculose, (13–)25–30(−40) (av. 28) μm long and (1.5–)2(−2.5) (av. 2) μm wide. Phialides terminal or lateral, mostly monophialidic, smooth to verruculose, pale brown to hyaline, collarettes, 1.5–3 μm long, 1–1.5 μm wide; type II phialides predominant, type I phialides subcylindrical, occasionally widened at the base, (4–)9–11(−17) × (1.5–)2(−2.5) (av. 10.5 × 2) μm; type II phialides subulate, navicular, or subcylindrical, attenuated at the base and tapering towards the apex, (8–)13–15(−20) × 1.5–2(−3) (av. 14 × 2) μm; type III phialides subcylindrical, subulate (20–)21–23(−25) × 2(−2.5) (av. 22 × 2) μm. Conidia hyaline, mostly allantoid, subcylindrical or cylindrical, ellipsoidal (2.5–)4(−5.5) × (1.5–)2 (av. 4 × 2) μm, L/W = 2. On surface or submerged in the agar: Phialides hyaline, subcylindrical, 4–9(−14) × 1(−2) (av. 6 × 1) μm. Conidia hyaline, subcylindrical or allantoid, 2.5–5(−8.5) × (1–)2(−3) (av. 5 × 2) μm, L/W = 2.5.
Cultural characteristics — Colonies attaining a radius of 8 mm after 8 d at 25 °C. Minimum temperature for growth 12 °C, optimum 33 °C, maximum 37 °C. Colonies on MEA flat, cottony, with entire margin; after 16 d, colonies pale brown to beige towards the edge above, pale orange in reverse. Colonies on PDA flat, short, woolly to felty, with entire edge, after 16 d colonies brown to beige, pale greyish orange and whitish towards the margin above, pale greyish sepia in reverse, becoming whitish towards the edge. Colonies on OA flat, felty, with entire margin; after 16 d pale orange to yellow towards the margin above, same in reverse, producing yellow pigmentation in the agar.
Substrate — Vitis vinifera.
Known distribution — Italy.
Specimen examined. Italy, Tuscany, San Gimignano, isolated from the margin of necrosis in the trunk of Vitis vinifera sampled from an about 100 yr old vineyard that showed wood esca symptoms, March 2007, L. Mugnai, holotype CBS H-20118, culture ex-type CBS 123033.
Notes — According to the phylogenetic analysis, Pm. tuscanum can be considered as a sister clade to Pm. iranianum. However, it can be distinguished from it by the production of coremium-like structures on MEA. These are fertile, erect hyphal bundles up to 2 mm tall and 1 mm wide, dark to pale brown, composed of conidiophores bearing conidia at the apex. Type II phialides are predominant in Pm. tuscanum. By comparison, Pm. iranianum lacks these structures and has a predominance of type III phialides.
DISCUSSION
The correct identification of fungi involved in diseases within the esca complex is a crucial precondition for the conduct of meaningful studies on the epidemiology of these destructive diseases of grapevine. Epidemiological studies will be especially important in the design of control strategies, since no fully effective chemical or biological control measures exist for this disease complex.
Several species of Phaeoacremonium have already been attributed to the grapevine diseases within the esca complex worldwide. However, the identity, distribution and frequency of the Phaeoacremonium species involved in many grapevine-growing areas, especially the area where Vitis vinifera evolved, have not yet been studied. The present study has included a wide collection of Phaeoacremonium isolates from Italy, mainly from isolated locations like Sardinia and Sicily where farmers grow local grape varieties. However, we also included a diverse set of isolates from other countries.
Integration of morphology, cultural characters and DNA sequence data revealed the presence of 13 Phaeoacremonium species in the areas sampled. Phylogenetic analyses of ACT and TUB sequences revealed that four of these species were novel. It is of interest to notice that the four new species here described were isolated from very old vines growing in Italy, Hungary and Croatia. Old vines were included in this study as a source of esca tracheomycotic fungi with the specific objective of gathering a population of both genera, Phaeomoniella and Phaeoacremonium, showing an as wide as possible variability within the population of the two fungi. Here are reported the results so far obtained in Phaeoacremonium.
Morphological traits such as presence or absence of hyphal warts, size of warts, conidiophore structure and cultural characteristics have been shown to be useful in species identification. For example, Pm. parasiticum can easily be distinguished from other species based on the occurrence of hyphal warts that are up to 3 μm diam (Mostert et al. 2006b). Some other species such as Pm. inflatipes and Pm. sphinctrophorum have frequently branched conidiophores, which can be used to distinguish them from species with short and infrequently branched or unbranched conidiophores. Nevertheless, species delimitation in this genus solely based on morphological and cultural characteristics has proven to be difficult. This difficulty is mainly inherent to the overlapping nature of morphological characters among the different species. Hence, DNA sequence data analysis remains of the utmost importance for complete and reliable species delineation. Mostert et al. (2006b) developed a multiple-entry polyphasic identification key for Phaeoacremonium species. This tool combines DNA sequence data, with different morphological and cultural characters to identify up to 22 Phaeoacremonium species. The four new species described in this study can be distinguished from the existing Phaeoacremonium species based on a combined cultural, morphological and DNA sequence dataset. Phaeoacremonium tuscanum clusters as sister clade to Pm. iranianum. However, it can be distinguished from Pm. iranianum by the production of coremium-like structures on MEA, consisting of erect, fertile hyphal bundles up to 2 mm high and 1 mm wide.
Two of the other new species described in this study, Pm. hungaricum and Pm. croatiense, cluster as sister clade to Pm. mortoniae and can only be distinguished by minute morphological differences. Phaeoacremonium hungaricum is distinct from Pm. mortoniae by the rare presence of conidiophores which are mostly reduced to conidiogenous cells or phialides. In fact, the aerial mycelium is composed of mostly elongated, ampulliform phialides, whereas Pm. croatiense can be differentiated from Pm. mortoniae based on cultural characters such as its olivaceous-grey colonies on MEA and the absence of yellow pigment production on OA. It is not surprising that these three species have a similar morphology as they have a close phylogenetic affinity, suggesting that they have evolved from a common ancestor.
The newly described Pm. sicilianum is markedly distinct from the other known Phaeoacremonium species. DNA sequence analysis showed that this clade is basal to the others, even though the species overlaps morphologically with Pm. parasiticum and Pm. inflatipes in its predominance of branched conidiophores. Phaeoacremonium parasiticum differs from Pm. sicilianum in its dark brown hyphae and large hyphal warts up to 3 μm diam. Phaeoacremonium sicilianum differs from Pm. inflatipes in its predominance of type I and II phialides and in its colony colour.
Growth temperature studies carried out for new species described in this study showed that Pm. tuscanum has a maximum growth temperature at 37–40 °C, reaching a radius of 7 mm after 8 d. This finding is quite interesting in relation to the ecology of Phaeoacremonium species, as several thermotolerant Phaeoacremonium species are associated with phaeohyphomycosis in humans (Mostert et al. 2005). Phaeoacremonium parasiticum, Pm. rubrigenum and Pm. inflatipes (later identified as Pm. alvesii), were initially reported from human phaeohyphomycosis (Padhye et al. 1998, Guarro et al. 2003). Phaeoacremonium krajdenii, Pm. parasiticum and Pm. venezuelense cause phaeohyphomycosis and have also been isolated from grapevines showing esca symptoms (Mostert et al. 2005). Four Phaeoacremonium species have heretofore been isolated only from human infections: Pm. amstelo-damense, Pm. griseorubrum, Pm. rubrigenum and Pm. tardicrescens (Mostert et al. 2005). The present study revealed that Pm. alvesii, griseorubrum and Pm. rubrigenum are also associated with grapevine. This finding further supports the hypothesis that human pathogenic Phaeoacremonium species may have originated from woody host plants. The same hypothesis has been proposed for some other groups of human opportunistic pathogens such as the black yeasts (de Hoog et al. 2007). To confirm this hypothesis, studies are needed inoculating strains from woody hosts into an animal model, and strains from human cases into woody plants.
Although several species of Phaeoacremonium are associated with necrosis and discolorations in grapevine wood, Pm. aleophilum is the most common species associated with foliar symptoms. It appears also to be the most widely distributed species (Crous et al. 1996, Larignon & Dubos 1997, Mugnai et al. 1999, Tegli et al. 2000, Mostert et al. 2006a) in grapevine. Our data were consistent with this pattern. Phaeoacremonium aleophilum was the most frequently isolated species with an incidence up to 80 % in all the samples examined. Inoculation studies have proven this species to be pathogenic on grapevines, showing that Pm. aleophilum can cause brown wood streaking (Adalat et al. 2000, Sparapano et al. 2000b, Feliciano et al. 2004, Halleen et al. 2005) and reduced shoot growth (Gubler et al. 2001), as well as esca symptoms on leaves and berries (Sparapano et al. 2000a, Feliciano et al. 2004).
A recent study has shown the occurrence of several novel Phaeoacremonium species on other woody hosts such as species in the genus Prunus (Damm et al. 2008). However, on grapevine the pathological relevance of the other Phaeoacremonium species as well as of the novel species described in this study remains to be determined.
The prevalence of the other 12 Phaeoacremonium species differs among grapevine growing areas. Phaeoacremonium griseorubrum, Pm. scolyti and Pm. viticola represent new records for Italy, Pm. mortoniae and Pm. rubrigenum for Croatia, Pm. alvesii for Turkey and Pm. mortoniae for Hungary. Other species that have also been isolated in relatively high frequencies from the different grapevine growing areas studied here include Pm. iranianum and Pm. mortoniae.
Where known, Phaeoacremonium species have teleomorphs in the genus Togninia (Diaporthales, Togniniaceae). Several researchers have induced the teleomorph of Phaeoacremonium species by crossing complementary strains in vitro (Mostert et al. 2005, 2006b, Damm et al. 2008). Our attempts to induce the teleomorph for the species described in this paper were unsuccessful.
Given the occurrence of various Phaeoacremonium species on grapevine and the involvement of some of those species in human infections, accurate identification is critical. Since, as mentioned, morphological identification is not always reliable, robust molecular-based detection tools are of great help in species detection and identification. As mentioned, an existing multiplex PCR assay based on use of species-specific TUB and ACT primers can identify 22 species of Phaeoacremonium (Mostert et al. 2006b). It is necessary to test these primer sets on DNA from the new species described here to determine if target specificity remains intact. The ACT and TUB gene barcodes generated in the present study can be used to develop species-specific molecular detection tools that will facilitate ecological and epidemiological studies.
Acknowledgments
The work of Salwa Essakhi, PhD student of the University of Florence, was in part financially supported by the European Commission’s Research Infrastructure Action via the SYNTHESYS Project (NL-TAF-4099), which is gratefully acknowledged. For providing material, we warmly acknowledge Salvatorica Serra from Sardinia, Giovanni Raiti, Pietro Rinaldi and Filippo Trovato from Sicily, Terézia Dula Bencene from Hungary, Bodgan Cvjetković from Croatia, Pietro Tsicopoulos and Dimitri Dimou from Greece, Saygili Hikmet from Turkey and Lisa van der Water from the USA. The first author thanks Dr Mahdi Arzanlou for technical help and scientific discussions. We also thank Tamara Cinelli for helping in the fungal isolation, Marjan Vermaas for preparing the photographic plates and Arien van Iperen for helping with the drying of culture specimens. The Italian part of the research study was commissioned from ARSIA-Toscana (Regional Agency for Development and Innovation in Agriculture and Forestry) by 14 administrative regions and one autonomous province, and financed with funds provided by the Ministero per le Politiche Agricole e Forestali (Ministry for Agriculture and Forestry Policy) to implement the inter-Regional Project ‘Grapevine esca: research and experiment in the nursery and in the field for prevention and cure.’
REFERENCES
- Adalat K, Whiting C, Rooney S, Gubler WD. 2000. Pathogenicity of three species of Phaeoacremonium spp. on grapevine in California. Phytopathologia Mediterranea 39: 92 – 99 [Google Scholar]
- Arzanlou M, Groenewald JZ, Gams W, Braun U, Shin H-D, Crous PW. 2007. Phylogenetic and morphotaxonomic revision of Ramichloridium and allied genera. Studies in Mycology 58: 57 – 93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calzarano F, Di Marco S. 2007. Wood discoloration and decay in grapevines with esca proper and their relationship with foliar symptoms. Phytopathologia Mediterranea 46: 96 – 101 [Google Scholar]
- Carbone I, Kohn LM. 1999. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91: 553 – 556 [Google Scholar]
- Crous PW, Gams W. 2000. Phaeomoniella chlamydospora gen. et comb. nov., a causal organism of Petri grapevine decline and esca. Phytopathologia Mediterranea 39: 112 – 118 [Google Scholar]
- Crous PW, Gams W, Wingfield MJ, Wyk PS van. 1996. Phaeoacremonium gen. nov. associated with wilt and decline diseases of woody hosts and human infections. Mycologia 88: 786 – 796 [Google Scholar]
- Damm U, Mostert L, Crous PW, Fourie PH. 2008. Novel Phaeoacremonium species associated with necrotic wood of Prunus trees. Persoonia 20: 87 – 102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J, Marchi G, Pascoe I. 2001. Young esca in Australia. Phytopathologia Mediterranea 40: S303 – S310 [Google Scholar]
- Edwards J, Pascoe I. 2004. Occurrence of Phaeomoniella chlamydospora and Phaeoacremonium aleophilum associated with Petri disease and esca in Australian grapevines. Australasian Plant Pathology 33: 273 – 279 [Google Scholar]
- Eskalen A, Rooney-Latham S, Gubler WD. 2005a. Occurrence of Togninia fraxinopennsylvanica on Esca diseased grapevines (Vitis vinifera) and declining ash trees (Fraxinus latifolia) in California vineyards. Plant Disease 89: 528 [DOI] [PubMed] [Google Scholar]
- Feliciano AJ, Eskalen A, Gubler WD. 2004. Differential susceptibility of three grapevine cultivars to Phaeoacremonium aleophilum and Phaeomoniella chlamydospora in California. Phytopathologia Mediterranea 43: 66 – 69 [Google Scholar]
- Fischer M. 2002. A new wood-decaying basidiomycete species associated with esca of grapevine: Fomitiporia mediterranea (Hymenochaetales). Mycological Progress 1: 315 – 324 [Google Scholar]
- Fischer M. 2006. Biodiversity and geographic distribution of basidiomycetes causing esca-associated white rot in grapevine: a worldwide perspective. Phytopathologia Mediterranea 45: S30 – S42 [Google Scholar]
- Gams W, Verkley GJM, Crous PW. (eds). 2007. CBS course of mycology Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands: [Google Scholar]
- Glass NL, Donaldson GC. 1995. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Applied and Environmental Microbiology 61: 1323 – 1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graniti A, Surico G, Mugnai L. 2000. Esca of grapevine: a disease complex or a complex of diseases? Phytopathologia Mediterranea 39: 16 – 20 [Google Scholar]
- Guarro J, Alves SH, Gené J, Grazziotin NA, Muzzuco R, Dalmagro C, Capilla J, Zaror L, Mayayo E. 2003. Two cases of subcutaneous infection due to Phaeoacremonium spp. Journal of Clinical Microbiology 41: 1332 – 1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler WD, Eskalen A, Feliciano AJ, Khan A. 2001. Susceptibility of grapevine pruning wounds to Phaeomoniella chlamydospora and Phaeoacremonium spp. Phytopathologia Mediterranea 40: S482 – S483 [Google Scholar]
- Halleen F, Mostert L, Crous PW. 2005. Pathogenicity testing of Phialophora, Phialophora-like, Phaeoacremonium and Acremonium species isolated from vascular tissues of grapevines. Proceedings of the 4th International Workshop on Grapevine Trunk Diseases, Stellenbosch, South Africa: 58 [Google Scholar]
- Hillis DM, Bull JJ. 1993. An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Systematic Biology 42: 182 – 192 [Google Scholar]
- Hoog GS de, Nishikaku AS, Fernandez-Zeppenfeldt G, GonzáPadín-lez C, Burger E, Badali H, Richard-Yegres N, Gerrits van den Ende AHG. 2007. Molecular analysis and pathogenicity of the Cladophialophora carrionii complex, with the description of a novel species. Studies in Mycology 58: 219 – 234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larignon P, Dubos B. 1997. Fungi associated with esca disease in grapevine. European Journal of Plant Pathology 103: 147 – 157 [Google Scholar]
- Marchi G, Peduto F, Mugnai L, Di Marco S, Calzarano F, Surico G. 2006. Some observations on the relationship of manifest and hidden esca to rainfall. Phytopathologia Mediterranea 45: S117 – S126 [Google Scholar]
- Martin MT, Cobos R. 2007. Identification of fungi associated with grapevine decline in Castilla y León (Spain). Phytopathologia Mediterranea 46: 18 – 25 [Google Scholar]
- Morton L. 2000. Viticulture and grapevine declines: lessons of black goo. Phytopathologia Mediterranea 39: 59 – 67 [Google Scholar]
- Mostert L, Groenewald JZ, Summerbell RC, Gams W, Crous PW. 2006b. Taxonomy and pathology of Togninia (Diaporthales) and its Phaeoacremonium anamorphs. Studies in Mycology 54: 1 – 115 [Google Scholar]
- Mostert L, Groenewald JZ, Summerbell RC, Robert V, Sutton DA, Padhye AA, Crous PW. 2005. Species of Phaeoacremonium associated with infections in humans and environmental reservoirs in infected woody plants. Journal of Clinical Microbiology 43: 1752 – 1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostert L, Hallen F, Fourie P, Crous PW. 2006a. A review of Phaeoacremonium species involved in Petri disease and esca of grapevines. Phytopathologia Mediterranea 45: S12 – S29 [Google Scholar]
- Mugnai L, Graniti A, Surico G. 1999. Esca (black measles) and brown wood-streaking: two old and elusive diseases of grapevines. Plant Disease 83: 404 – 416 [DOI] [PubMed] [Google Scholar]
- O’Donnell K, Cigelnik E. 1997. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Molecular Phylogenetics and Evolution 7: 103 – 116 [DOI] [PubMed] [Google Scholar]
- Padhye AA, Davis MS, Baer D, Reddick A, Sinha KK, Ott J. 1998. Phaeohyphomycosis caused by Phaeoacremonium inflatipes. Journal of Clinical Microbiology 36: 2763 – 2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page RDM. 1996. TREEVIEW: An application to display phylogenetic trees on personal computers. Computer Applications in the Biosciences 12: 357 – 358 [DOI] [PubMed] [Google Scholar]
- Pascoe I, Cottral E. 2000. Developments in grapevine trunk diseases research in Australia. Phytopathologia Mediterranea 39: 68 – 75 [Google Scholar]
- Rambaut A. 2002. Sequence Alignment Editor. Version 2.0 Department of Zoology, University of Oxford, Oxford, United Kingdom: [Google Scholar]
- Rayner RW. 1970. A mycological colour chart. Commonwealth Mycological Institute and British Mycological Society; Kew, Surrey, United Kingdom: [Google Scholar]
- Schubert K, Braun U, Groenewald JZ, Crous PW. 2007. Cladosporium leaf-blotch and stem rot of Paeonia spp. caused by Dichloclasporium chlorocephalum gen. nov. Studies in Mycology 58: 95 – 104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparapano L, Bruno G, Ciccarone C, Graniti A. 2000a. Infection of grapevines by some fungi associated with esca. I. Fomitiporia punctata as wood-rot inducer. Phytopathologia Mediterranea 39: 46 – 52 [Google Scholar]
- Sparapano L, Bruno G, Graniti A. 2000b. Infection of grapevines by some fungi associated with esca. II. Interaction among Phaeoacremonium chlamydosporum, P. aleophilum and Fomitiporia punctata. Phytopathologia Mediterranea 39: 53 – 58 [Google Scholar]
- Surico G. 2001. Towards commonly agreed answers to some basic questions on esca. Phytopathologia Mediterranea 40: S487 – S490 [Google Scholar]
- Surico G, Mugnai L, Marchi G. 2006. Older and more recent observations on esca: a critical overview. Phytopathologia Mediterranea 45: S68 – S86 [Google Scholar]
- Swofford DL. 2003. PAUP*. Phylogenetic Analysis Using Parsimony (*and other methods). Version 4.0b10 Sinauer Associates, Sunderland, MA, USA: [Google Scholar]
- Tegli S, Santilli E, Bertelli E, Surico G. 2000. Genetic variation within Phaeoacremonium aleophilum and P. chlamydosporum in Italy. Phytopathologia Mediterranea 39: 125 – 133 [Google Scholar]
- Vasquez SJ, Gubler WD, Leavitt GM. 2007. Economic loss in California’s table grape vineyards due to measles. Phytopathologia Mediterranea 46: 118 [Google Scholar]