Table 1.
Comparison of Nicotine, Epibatidine, and Varenicline Radioligand Binding and Antinociception Data to 3′-(Substituted Phenyl)epibatidine Analogues (5a–m)
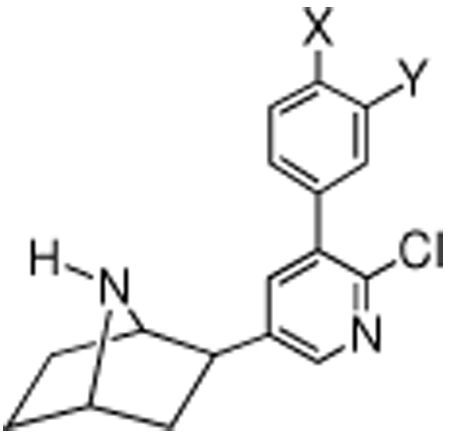 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| compound | X | Y | αβ [3H]epibatidine (Ki, nM) |
α7 [125I]iodoMLA (Ki, nM)b |
ED50 mg/kg tail-flickc |
ED50 mg/kg hot-platec |
ED50 mg/kg hypothermiac |
ED50 mg/kg spontaneous activityc |
AD50 (µg/kg)c |
|
| tail-flick | hot-plate | |||||||||
| (+)-epibatidine [(+)-1]d |
0.026 ± 0.002e | 198 | 0.0061e | 0.004e | 0.004e | 0.001e | ||||
| (−)-epibatidine [(−)-1]d |
0.018 ± 0.001e | 0.0066e | ||||||||
| nicotine (2)e |
1.5 ± 0.3 | 1.3 | 0.65 | 1.0 | 0.5 | |||||
| varenicline (3)f |
0.12 ± 0.02 | 32.5 ± 1.3 | 11% @ 10 | 10% @ 10 | 2.8 | 2.1 | 0.2 | 470 | ||
| 5a | H | H | 0.021 ± 0.005 | 260 ± 5.0 | 0.7g (0.5–1.0) |
1.0g (0.5–2.0) |
0.4g (0.1–0.9) |
0.35g (0.2–0.85) |
280 (80–900) |
30% @ 10,000 |
| 5b | F | H | 0.017 ± 0.003 | 309 ± 13 | 2.48 (1.7–3.5) |
1.27 (0.8–1.9) |
0.33 (0.22–0.67) |
0.33 (0.15–0.7) |
0.5 (0.06–29) |
30% @ 100 |
| 5c | H | F | 0.012 ± 0.001 | 250 ± 44 | 1.0 (0.7–1.5) |
0.43 (0.2–0.94) |
0.19 (0.1–0.7) |
0.07 (0.05–0.10) |
3.9 (5–30) |
86 (20–300) |
| 5d | Cl | H | 0.039 ± 0.005 | 209 ± 50 | 2.6 (2.3–3.3) |
2 (1.4–2.7) |
0.89 (0.5–1.2) |
0.53 (0.3–0.9) |
1 (0.1–27) |
10% @100 |
| 5e | H | Cl | 0.013 ± 0.001 | 30.5 ± 84 | 3.4 (2.6–4.3) |
4.2 (1.5–11.6) |
0.38 (0.2–1) |
0.06 (0.02–0.13) |
2.2 (4–15) |
80 (30–200) |
| 5f | H | Br | 0.016 ± 0.003 | 85 ± 10 | 0.54 (0.44–0.67) |
2.2 (1.2–3.8) |
0.67 (0.5–0.9) |
0.11 (0.06–0.22) |
10 (7–90) |
220 (50–600) |
| 5g | NO2 | H | 0.015 ± 0.001 | 250 ± 25 | 0.46 (0.33–0.64) |
0.38 (0.2–0.5) |
0.23 (0.1–0.6) |
0.24 (0.1–0.5) |
7 (3–10) |
17% @ 50 |
| 5h | H | NO2 | 0.008 ± 0.0003 | 229 ± 43 | 4.6 (3.3–6.5) |
3.2 (2.4–4.5) |
1.4 (1–2.4) |
0.9 (0.7–1.2) |
0.25 (0.02–0.7) |
180 (29–1078) |
| 5i | NH2 | H | 0.034 ± 0.001 | 234 ± 17 | 5 (3.4–7.2) |
4.5 (3.6–5.7) |
1.1 (0.8–1.9) |
0.52 (0.11–2.3) |
6 (5–7) |
0% @100 |
| 5j | H | NH2 | 0.017 ± 0.001 | 256 ± 46 | 0.34 (0.24–0.46) |
0.3 (0.17–0.53) |
0.31 (0.2–0.5) |
0.03 (0.009–0.12) |
0.14 (0.05–0.4) |
26 (5–90) |
| 5k | CH3O | H | 0.022 ± 0.008 | 175 ± 10 | 2.36 (2–3.7) |
1.1 (0.5–2.7) |
0.79 (0.55–1.1) |
0.43 (0.1–1.3) |
10 (1–9) |
10% @ 200 |
| 5l | H | CH3O | 0.019 ± 0.005 | 558 ± 50 | 0.7 (0.3–1.2) |
0.8 (0.3–1.7) |
0.7 (0.5–1.1) |
0.2 (0.18–0.23) |
29 (10–60) |
0% @100 |
| 5m | H | (CH3)2N | 0.009 ± 0.001 | 1100 ± 157 | 0% @ 2 | 0% @ 2 | 3.3 (2.1–5.6) |
2.0 (0.7–5.8) |
20 (10–50) |
560 (100–9200) |
All epibatidine analogues were tested as hydrochloride salts, and all were racemates.
Data represent means ± SE from at least three independent experiments.
Results are provided as ED50 or AD50 values (± confidence limits) or as a percent effect at the individual dose.
Compound (+)-1 is the natural epibatidine hydrochloride, and (−)-1 is the enantiomeric epibatidine hydrochloride.
Data taken from Ref. 15.
Data taken from Ref. 19.
Data taken from Ref. 13.
