Abstract
Pathophysiologic hypotheses for Alzheimer’s disease (AD) are centered on the role of the amyloid plaque Aβ peptide and the mechanism of its derivation from the amyloid precursor protein (APP). As part of the disease process, an aberrant axonai sprouting response is known to occur near Aβ deposits. A Nogo to Nogo-66 receptor (NgR) pathway contributes to determining the ability of adult CNS axons to extend after traumatic injuries. Here, we consider the potential role of NgR mechanisms in AD. Both Nogo and NgR are mislocalized in AD brain samples. APP physically associates with the NgR. Overexpression of NgR decreases Aβ production in neuroblastoma culture, and targeted disruption of NgR expression increases transgenic mouse brain Aβ levels, plaque deposition, and dystrophic neurites. Infusion of a soluble NgR fragment reduces Aβ levels, amyloid plaque deposits, and dystrophic neurites in a mouse transgenic AD model. Changes in NgR level produce parallel changes in secreted APP and AB, implicating NgR as a blocker of secretase processing of APP. The NgR provides a novel site for modifying the course of AD and highlights the role of axonal dysfunction in the disease.
The molecular pathophysiology of Alzheimer’s Disease (AD) has centered on the β-cleavage of amyloid precursor protein and n the deposition of Aβ in plaques [1]. Disruption of synaptic activity by Aβ oligomers are thought to be central in the disease [2, 3]. Chonic alterations of synaptic function may in turn lead to the aberrant sprouting which surrounds amyloid plaques as “dystrophic neurites”, one of the neuroanatomical hallmarks of AD [4].
We have studied the role of Nogo to Nogo-66 Receptor (NgR) signaling pathway in limiting neuronal plasticity after trauma to the adult central nervous system [5]. We have reported that disruption of the NgR pathway alleviates central nervous system myelin inhibition and promotes neuronal outgrowth and plasticity [6, 7]. Because the molecular mechanisms for sprouting after mechanical trauma and in Alzheimer’s disease may overlap, we have examined the role of NgR in AD [8]. We highlight NgR’s role as a surface neuronal molecule that modulates APP/Aβ metabolism, and that may participate in Aβ pathology.
Previous work has identified several cell surface molecules that might mediate Aβ effects on the neuron. Aβ-binding proteins include the receptor for advanced glycation end products (RAGE) [9], the low-affinity NGF receptor (p75-NTR)[10] and nicotinic acetylcholine receptors [11–13]. These Aβ binding partners have been implicated in certain Aβ cellular effects including cell death, altered synaptic transmission, stimulation of neurite outgrowth and inhibition of neurite outgrowth [14, 15]. However, signal transduction from these cell surface receptors to Alzheimer’s pathology requires further study with respect to ligand binding characteristics (each study measured Aβ affinities differently), in vivo loss of function outcomes (only a dominant negative RAGE model has been reported in [16]) and relevance to synaptic transmission.
To explore NgR’s ability to act as a cellular binding site for Aβ, we employed the alkaline-fusion phosphatase (AP) method that has been successfully employed to identify receptors such as Eph, Neuropilin, Neogenin and NgR [5, 17–20]. The sensitivity and ease of the alkaline phosphatase method make it an attractive technique for characterizing cell surface interactions. We assayed the binding of AP-Aβ and AP-APP fusion proteins to transfected COS-7 cells by methods employed for other ligands [5, 18–22]. We demonstrated substantial affinity of AP-Aβ for NgR-expressing COS-7 cells, but not for cells expressing RAGE, p75 or NgR3 [8].
Since both AP-ecto-APP and AP-Aβ bind to NgR, we assessed APP and NgR co-immunprecipitation [8]. In rat brain, APP physically associates with the NgR. Since NgR is a GPI-linked protein and enriched in lipid rafts, it is appropriate to note that APP processing has been reported to occur in lipid rafts [23]. The data suggest that the presence of NgR in lipid rafts may limit BACE cleavage of APP. In neuroblastoma cell culture, overexpression of NgR decreases Aβ production [8], an effect that may occur by sequestering APP from secretase activity.
To examine the significance of the NgR/APP interaction on processing in vivo, Alzheimer transgenic mice [24, 25] were bred onto a NgR null background. Compared to control, the absence of NgR increases the accumulation of both Aβ plaque and immunoreactive Aβ about two-fold [8]. Both Aβ(1–40) and Aβ(1–42) increase, suggesting that γ-secretase preference is not altered by NgR. There is a parallel two-fold increase in neuritic dystrophy in the NgR −/− animals. The data show that endogenous NgR has a role in restricting brain Aβ accumulation.
To enhance NgR/APP interactions in brain, soluble NgRecto-Fc protein was infused intracerebroventricularly into Alzheimer transgenic mice from 6–8 months of age mice [8]. In the NgRecto-Fc treated mice, the deposition of Aβ into plaque is reduced by 50% in the brain. The Aβ40 to Aβ42 ratio is not altered in the treated group. The prevalence of dystrophic neurites was also reduced by half in the treated mice. Thus, excess NgR protein reduces pathology in FAD transgenic mice.
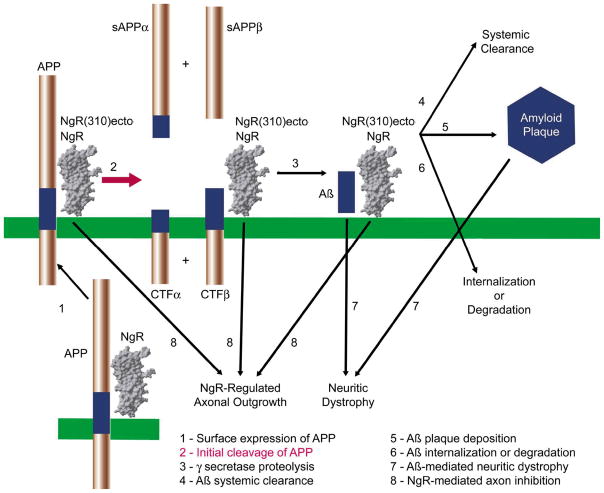
There are several sites in APP/Aβ metabolism at which NgR binding might regulate APP trafficking or APP proteolysis or Aβ metabolism or Aβ deposition or Aβ toxic effects, as illustrated in Figure 1. Overall, our in vivo data indicate that there is an inverse relationship between NgR levels and Aβ levels. To the extent that NgR is acting upstream in these cascades, secreted APP fragments are expected to change in parallel with Aβ levels. Since we found that sAPPα and sAPPs levels in brain changed in the same direction as Aβ levels [8], there is some evidence for uptstream effects of NgR on APP metabolism to peptide derivatives.
Figure 1. Mechanism of NgR interaction with APP/Aβ metabolism in AD.
A schematic illustrates the interaction of GPI-anchored endogenous NgR and exogenous soluble NgR(310)ecto-Fc with APP, with β-CTF fragments of APP and with Aβ. NgR association with APP appears to reduce access of α- and β-secretases to APP, as indicated in red thick arrow. Additional steps that may be altered by NgR are listed in black letters and indicated with thin black arrows. [8].
Anatomical examination was employed to assess the potential relevance of NgR for human AD pathophysiology. In one recent study, Nogo-A is overexpressed by hippocampal neurons in AD and is associated with β-amyloid deposits in senile plaques [26]. In AD brain sections, we found that both Nogo and NgR are mislocalized [8]. In all of the AD cases, Nogo-A is shifted to a neuronal perikaryal localization. While control cases exhibit the highest concentration of the NgR protein in neuronal cell bodies, NgR is shifted to the neuropil of AD brain. A fraction of NgR is also concentrated in amyloid plaques. The altered distribution of Nogo and NgR in AD brain are consistent with either a primary or a secondary role in the pathology. Although no studies have yet reported an association of Nogo or NgR genetic variation with AD, the current data provide a rationale to consider these loci.
In conclusion, NgR provides a novel site for modifying the course of AD. The infusion of soluble NgR fragment reduces Aβ levels, amyloid plaque deposits and dystrophic neurites in a mouse transgenic AD model [8]. Endogenous NgR interacts with APP and Aβ to limit Aβ accumulation in vivo. It remains to be determined whether NgR may also play a role in mediating some of the deleterious actions of Aβ peptide. These studies on NgR highlight the role of axonal function and dysfunction in AD.
Acknowledgments
This work is supported by grants from the Insitute for the Study of Aging and the N.I.H. to S.M.S. and by an institutional N.I.H. Medical Scientist Training Grant to J.H.P. S.M.S. is a member of the Kavli Institute of Neuroscience at Yale.
References
- 1.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002 Jul 19;297(5580):353–6. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 2.Lesne S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, et al. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006 Mar 16;440(7082):352–7. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- 3.Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, et al. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002 Apr 4;416(6880):535–9. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 4.Guevara J, Dilhuydy H, Espinosa B, Delacourte A, Quirion R, Mena R, et al. Coexistence of reactive plasticity and neurodegeneration in Alzheimer diseased brains. Histol Histopathol. 2004 Oct;19(4):1075–84. doi: 10.14670/HH-19.1075. [DOI] [PubMed] [Google Scholar]
- 5.Fournier AE, GrandPre T, Strittmatter SM. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature. 2001 Jan 18;409(6818):341–6. doi: 10.1038/35053072. [DOI] [PubMed] [Google Scholar]
- 6.McGee AW, Yang Y, Fischer QS, Daw NW, Strittmatter SM. Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science. 2005 Sep 30;309(5744):2222–6. doi: 10.1126/science.1114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.GrandPre T, Li S, Strittmatter SM. Nogo-66 receptor antagonist peptide promotes axonal regeneration. Nature. 2002 May 30;417(6888):547–51. doi: 10.1038/417547a. [DOI] [PubMed] [Google Scholar]
- 8.Park JH, Gimbel DA, GrandPre T, Lee JK, Kim JE, Li W, et al. Alzheimer precursor protein interaction with the Nogo-66 receptor reduces amyloid-beta plaque deposition. J Neurosci. 2006 Feb 1;26(5):1386–95. doi: 10.1523/JNEUROSCI.3291-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang X, Hyder F, Shulman RG. Activation of single whisker barrel in rat brain localized by functional magnetic resonance imaging. Proc Natl Acad Sci U S A. 1996 Jan 9;93(1):475–8. doi: 10.1073/pnas.93.1.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuner P, Schubenel R, Hertel C. Beta-amyloid binds to p57NTR and activates NFkappaB in human neuroblastoma cells. J Neurosci Res. 1998 Dec 15;54(6):798–804. doi: 10.1002/(SICI)1097-4547(19981215)54:6<798::AID-JNR7>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 11.Wang HY, Lee DH, D’Andrea MR, Peterson PA, Shank RP, Reitz AB. beta-Amyloid(1–42) binds to alpha7 nicotinic acetylcholine receptor with high affinity. Implications for Alzheimer’s disease pathology. J Biol Chem. 2000 Feb 25;275(8):5626–32. doi: 10.1074/jbc.275.8.5626. [DOI] [PubMed] [Google Scholar]
- 12.Dineley KT, Westerman M, Bui D, Bell K, Ashe KH, Sweatt JD. Beta-amyloid activates the mitogen-activated protein kinase cascade via hippocampal alpha7 nicotinic acetylcholine receptors: In vitro and in vivo mechanisms related to Alzheimer’s disease. J Neurosci. 2001 Jun 15;21(12):4125–33. doi: 10.1523/JNEUROSCI.21-12-04125.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagele RG, D’Andrea MR, Anderson WJ, Wang HY. Intracellular accumulation of beta-amyloid(1–42) in neurons is facilitated by the alpha 7 nicotinic acetylcholine receptor in Alzheimer’s disease. Neuroscience. 2002;110(2):199–211. doi: 10.1016/s0306-4522(01)00460-2. [DOI] [PubMed] [Google Scholar]
- 14.Kamenetz F, Tomita T, Hsieh H, Seabrook G, Borchelt D, Iwatsubo T, et al. APP processing and synaptic function. Neuron. 2003 Mar 27;37(6):925–37. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- 15.Walsh DM, Klyubin I, Fadeeva JV, Rowan MJ, Selkoe DJ. Amyloid-beta oligomers: their production, toxicity and therapeutic inhibition. Biochem Soc Trans. 2002 Aug;30(4):552–7. doi: 10.1042/bst0300552. [DOI] [PubMed] [Google Scholar]
- 16.Arancio O, Zhang HP, Chen X, Lin C, Trinchese F, Puzzo D, et al. RAGE potentiates Abeta-induced perturbation of neuronal function in transgenic mice. Embo J. 2004 Oct 13;23(20):4096–105. doi: 10.1038/sj.emboj.7600415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flanagan JG, Cheng HJ, Feldheim DA, Hattori M, Lu Q, Vanderhaeghen P. Alkaline phosphatase fusions of ligands or receptors as in situ probes for staining of cells, tissues, and embryos. Methods Enzymol. 2000;327:19–35. doi: 10.1016/s0076-6879(00)27264-9. [DOI] [PubMed] [Google Scholar]
- 18.Rajagopalan S, Deitinghoff L, Davis D, Conrad S, Skutella T, Chedotal A, et al. Neogenin mediates the action of repulsive guidance molecule. Nat Cell Biol. 2004 Aug;6(8):756–62. doi: 10.1038/ncb1156. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi T, Fournier A, Nakamura F, Wang LH, Murakami Y, Kalb RG, et al. Plexin-neuropilin-1 complexes form functional semaphorin-3A receptors. Cell. 1999 Oct 1;99(1):59–69. doi: 10.1016/s0092-8674(00)80062-8. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura F, Tanaka M, Takahashi T, Kalb RG, Strittmatter SM. Neuropilin-1 extracellular domains mediate semaphorin D/III-induced growth cone collapse. Neuron. 1998 Nov;21(5):1093–100. doi: 10.1016/s0896-6273(00)80626-1. [DOI] [PubMed] [Google Scholar]
- 21.Fournier AE, Gould GC, Liu BP, Strittmatter SM. Truncated soluble Nogo receptor binds Nogo-66 and blocks inhibition of axon growth by myelin. J Neurosci. 2002 Oct 15;22(20):8876–83. doi: 10.1523/JNEUROSCI.22-20-08876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.GrandPre T, Nakamura F, Vartanian T, Strittmatter SM. Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature. 2000 Jan 27;403(6768):439–44. doi: 10.1038/35000226. [DOI] [PubMed] [Google Scholar]
- 23.Cordy JM, Hooper NM, Turner AJ. The involvement of lipid rafts in Alzheimer’s disease. Mol Membr Biol. 2006 Jan-Feb;23(1):111–22. doi: 10.1080/09687860500496417. [DOI] [PubMed] [Google Scholar]
- 24.Borchelt DR, Ratovitski T, van Lare J, Lee MK, Gonzales V, Jenkins NA, et al. Accelerated amyloid deposition in the brains of transgenic mice coexpressing mutant presenilin 1 and amyloid precursor proteins. Neuron. 1997 Oct;19(4):939–45. doi: 10.1016/s0896-6273(00)80974-5. [DOI] [PubMed] [Google Scholar]
- 25.Jankowsky JL, Xu G, Fromholt D, Gonzales V, Borchelt DR. Environmental enrichment exacerbates amyloid plaque formation in a transgenic mouse model of Alzheimer disease. J Neuropathol Exp Neurol. 2003 Dec;62(12):1220–7. doi: 10.1093/jnen/62.12.1220. [DOI] [PubMed] [Google Scholar]
- 26.Gil V, Nicolas O, Mingorance A, Urena JM, Tang BL, Hirata T, et al. Nogo-A expression in the human hippocampus in normal aging and in Alzheimer disease. J Neuropathol Exp Neurol. 2006 May;65(5):433–44. doi: 10.1097/01.jnen.0000222894.59293.98. [DOI] [PubMed] [Google Scholar]