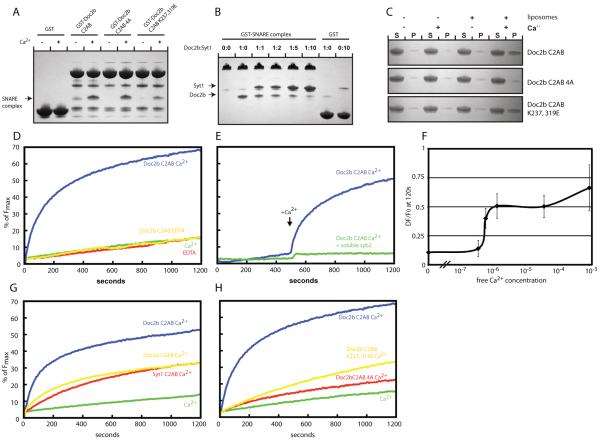
Fig. 4.
SNARE complex binding and promotion of SNARE-dependent membrane fusion by Doc2b. (A) Coomassie stained gels showing a pull down experiment in the presence or absence of Ca2+ using the indicated Doc2b fusion proteins as bait and the purified SNARE complex as prey. The co-pelleted SNARE complex is indicated by an arrow. (B) Coomassie stained gels showing a pull down in the presence of Ca2+ using the GST-SNARE complex as bait and the Doc2b and synaptotagmin-1 C2AB domains as prey. GST served as control for unspecific binding by the C2AB domains of Doc2B and synaptotagmin-1, respectively. The co-pelleted Doc2B and synaptotagmin-1 C2AB domains are indicated by arrows. (C) Liposome co-sedimentation assay in the presence or absence of Ca2+ using the indicated proteins and liposomes composed of 20% PS, 70% PC and 10% cholesterol. (D) In vitro membrane fusion assay using reconstituted full length SNAREs in the absence or presence of Ca2+ and/or 7.5 μM Doc2b C2AB. (E) In vitro membrane fusion assay as in (D) but in the presence or absence of the soluble SNARE domain of synaptobrevin. Ca2+ was added at 500 s. (F) Ca2+ dose-dependence curve showing the change of fluorescence at 120 s in the reconstituted fusion assay in the presence of 7.5 μM Doc2b. Doc2b efficiently promotes fusion at sub-μM Ca2+ concentrations. The graph summarizes data from 3 independent experiments. (G) Comparison of the fusion promoting activities of the Doc2b, Doc2a and synaptotagmin-1 C2AB domains. (H) Fusion experiment using the indicated Doc2b C2AB proteins reveals that Ca2+-dependent membrane and SNARE-binding are required for efficient fusion promotion. All data shown are representatives of at least 3 independent experiments.